The Elderly and Aging
Karen P. Alexander
Christopher M. O’Connor
Overview
The extraordinary increase in older persons, coupled with their high prevalence of cardiovascular disease, makes understanding the age-related changes in the cardiovascular system and disease manifestations in the elderly an important endeavor. According to the American College of Cardiology, the percentage of elderly patients treated per cardiologist will continue to increase through the year 2020 (1). Demand for services coupled with the high cost of cardiovascular care necessitates the careful consideration of treatment benefits across patient age, and the heterogeneity of aging requires careful consideration of treatment risks.
Although randomized controlled trials provide treatment-specific information, they do not clarify the value of treatments applied to populations differing from those studied, like the elderly. Age-related changes in the cardiovascular system modify presentation and outcome of cardiovascular disease, and age-related changes in other systems alter the pharmacokinetics and pharmacodynamics of cardiovascular drugs. In addition, treatments known to be effective, safe, and inexpensive are often underused (e.g., aspirin and β-blockers), while others with less evidence to clarify their risk and expense are used frequently (e.g., catheter-based interventions). Because patient-centered evidence-based medicine in the elderly is of enormous importance, more information on drug efficacy and safety as well as functional and health status outcomes across age is needed. This broader perspective along with consideration of patient preferences should guide treatment selection from among evidence-supported options. In so doing, cardiologists can provide the best cardiovascular care within the global health context of the elderly patient.
Historical Perspective
Never has a society had as many elderly, nor committed so large a share of community resources to their well-being. The absolute and proportional number of older Americans in the population is increasing at a remarkable rate (Fig. 33.1). Since the beginning of the twentieth century, longevity has increased by about 30 years (2). In 1900, life expectancy in the United States at birth was 47 years. In 2002, life expectancy at birth in the United States reached a high of 77.3 years (3). Despite
longer life expectancies, maximum life span remains limited at 85 to 90 years (4,5). The proportion of people aged 65 years or older is projected to increase from 12.4% to 19.6% in the United States between 2000 and 2030 (6). During this same time interval the absolute number of the oldest old in the United States—age 85 years or older—will increase most dramatically, doubling from 9.3 million to 19.5 million. Thus, it is the larger number of individuals living into later life that accounts for the current demographic shift. The key outcome for treatment of cardiovascular disease in the elderly has shifted to a focus on compression of morbidity.
longer life expectancies, maximum life span remains limited at 85 to 90 years (4,5). The proportion of people aged 65 years or older is projected to increase from 12.4% to 19.6% in the United States between 2000 and 2030 (6). During this same time interval the absolute number of the oldest old in the United States—age 85 years or older—will increase most dramatically, doubling from 9.3 million to 19.5 million. Thus, it is the larger number of individuals living into later life that accounts for the current demographic shift. The key outcome for treatment of cardiovascular disease in the elderly has shifted to a focus on compression of morbidity.
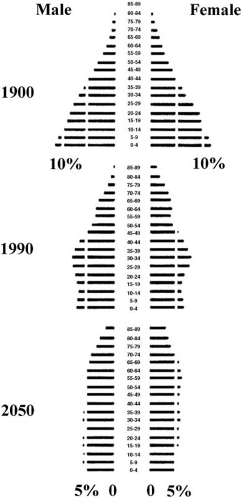 FIGURE 33.1. Distribution of the U.S. population by 5-year age categories for 1900 and 1990, and projections for 2050 (6). The distribution becomes rectangular as the proportion of the population achieving old age increases and (projected) birth rates stabilize. (Source: From U.S. Census Bureau. J NIH Res 1995;7:29. ) |
More than 40% of the annual U.S. budget is spent on Medicare and Medicaid (7). Congestive heart failure (CHF) is the most common diagnosis-related group in the Medicare population (8). In addition, 60% of hospital admissions for myocardial infarction (MI) are among Medicare eligible patients (age ≥65) (9). Atrial fibrillation occurs at a median age of 75 years, and is present in 9% of the population between ages 80 and 89 years (10,11). Not surprisingly, 25.8% of elderly nursing home residents age 65 or older had a primary cardiovascular diagnosis at the time of admission to long-term care (8).
Despite the greater burden of disease in the elderly, cardiovascular therapies have primarily been studied in younger populations. Until recently, older patients were explicitly excluded from therapeutic trials (12). Accordingly, the average age in heart failure trials ranges from 59 to 64 years (13,14,15). In addition, fewer than 20% of elderly dwelling in the community with heart failure would meet other inclusion criteria for trials, resulting in limited information on CHF in this population (16). The average age in acute coronary syndrome (ACS) trials ranges from 57 to 62 years, also representing a younger and healthier population than their community treated counterparts (17,18,19,20,21). The data emphasize that the medical system spends the least money studying cardiovascular patients who account for the greatest health care expenditures.
In this chapter, the diagnosis and management of cardiovascular disease in the elderly is addressed considering three key concepts. First, age-related cardiovascular changes, including degenerative changes in the conduction system, alterations in the myocardium and left ventricular (LV) function, and senile calcification of the aortic valve, are discussed. These and other changes are the consequences of the aging process, but substantially alter the clinical presentation and course of cardiovascular disease. Second, consideration of altered pharmacokinetics and pharmacodynamics related to patient age are discussed. The third issue is the heterogeneity of the aging process, which dictates the need for highly individualized decisions in the elderly, particularly in those beyond age 75 years. For many older persons, quality of life and functional independence, rather than longevity, become primary therapeutic targets. To integrate these subjective aspects of care into the patient’s entire health context requires substantial time and attention. The charge of cardiovascular health care providers with regard to the aging population must be to develop best approaches to cardiovascular care that exists, which optimizes quality of life within the entire health context of the elderly individual.
The Older Cardiac Patient
Cardiovascular Changes Related to Aging
Age-related changes in the cardiovascular system parallel age-related changes elsewhere in the body. However, age-related changes in a single organ or group of organs, such as the brain, lungs, or kidneys, may predominate, while, other organs remain unaffected. Age-related changes in the cardiovascular system are specific and enumerable (Tables 33.1 and 33.2). First, elderly hearts experience myocyte hypertrophy along with an increase in the connective tissue matrix (22). On the cellular level, total number of myocytes decreases and remaining myocytes hypertrophy (23). The weight of the heart increases 1.5 g/year between 30 and 90 years of age because of this hypertrophy and connective tissue deposition (24,25). With aging, ventricular chamber dimensions decrease with septal hypertrophy because of increased ventricular septal thickness and decreased base-to-apex dimensions. The “sigmoid septum” of aging is another morphologic manifestation of the senescent heart resulting from the reduction in the ventricular cavity
size and rightward shift of the ascending aorta (26). Although not hemodynamically significant, this curved septum simulates asymmetric hypertrophic cardiomyopathy. Despite structural changes, systolic function of the heart at rest remains essentially normal. However, alterations that impair peak systolic function do occur at the subcellular level of myocardial function. These include availability of energy stores, intracellular calcium handling, and transmembrane action potential (27). Changes in receptor density, receptor coupling, and postreceptor mechanisms reduce the response of myocardial cells to β-adrenergic stimulation with age (28). Intriguing studies in isolated cardiac muscles from older animals show a reduction in inotropic response to catecholamines and digitalis (which require a receptor) but an unchanged response of myofibrils to direct exposure to calcium (after chemical removal of the cell membrane). The altered pattern of Ca2+ regulation allows the myocardium of older hearts to generate force for a longer period of time following excitation (29). This enables the continued ejection of blood during late systole, a beneficial adaptation with respect to enhanced vascular stiffness and early reflected pulse waves. Older persons have higher levels of catecholamines and greater release of catecholamines with stress, but reduced chronotropic and inotropic responses.
size and rightward shift of the ascending aorta (26). Although not hemodynamically significant, this curved septum simulates asymmetric hypertrophic cardiomyopathy. Despite structural changes, systolic function of the heart at rest remains essentially normal. However, alterations that impair peak systolic function do occur at the subcellular level of myocardial function. These include availability of energy stores, intracellular calcium handling, and transmembrane action potential (27). Changes in receptor density, receptor coupling, and postreceptor mechanisms reduce the response of myocardial cells to β-adrenergic stimulation with age (28). Intriguing studies in isolated cardiac muscles from older animals show a reduction in inotropic response to catecholamines and digitalis (which require a receptor) but an unchanged response of myofibrils to direct exposure to calcium (after chemical removal of the cell membrane). The altered pattern of Ca2+ regulation allows the myocardium of older hearts to generate force for a longer period of time following excitation (29). This enables the continued ejection of blood during late systole, a beneficial adaptation with respect to enhanced vascular stiffness and early reflected pulse waves. Older persons have higher levels of catecholamines and greater release of catecholamines with stress, but reduced chronotropic and inotropic responses.
TABLE 33.1 Age-Related Changes in Cardiac Anatomy | ||||||
|---|---|---|---|---|---|---|
|
TABLE 33.2 Age-Related Changes in Cardiovascular Physiology | |||||
|---|---|---|---|---|---|
|
In contrast to systolic function, which is preserved at rest, diastolic function is impaired. Connective tissue matrix becomes replaced with a less distensible form of collagen. This causes greater stiffness of the senescent heart, requiring greater filling pressures to adapt via the Frank–Starling mechanism (30). Progressive cellular disarray, myocyte asynchrony, and abnormal calcium handling further affect the compliance and filling parameters during diastole. From a study of senescent animal models, the most predictable change in cardiac muscle function is longer duration of relaxation. This impaired relaxation with senescence is attributable to slower intracellular handling of calcium and longer action potentials, in addition to the stiffness from altered collagen. Echocardiographic Doppler studies in humans confirm prolonged relaxation and slower early diastolic filling with aging (31). However, end-systolic volumes are usually maintained by augmentation of late diastolic filling evidenced by exaggerated A wave and altered E:A ratio through the mitral valve (32). Diastolic function of the aging heart may be worsened by coexisting structural changes, such as mitral or aortic valvular disease, hypertension, atrial arrhythmias, or senile amyloidosis, which further alter hemodynamic conditions.
Age-related changes in the arterial system begin in the 30s and accelerate through midlife. Increased collagen deposition and weakened vascular elastin result in altered elasticity, distensibility, and dilatation. These changes, particularly in the intima, appear to resemble those that occur during atherosclerosis (33,34). Within the vascular media, there is progressive growth of smooth muscle, as well as deposition of lipids and calcium in the elastic lamella. Stiffening of the central arteries results in higher pulse wave velocities and augmentation in systolic arterial pressure, and whereas the lower elasticity results in a diminished contribution of arterial recoil to forward arterial perfusion. As arterial distensibility decreases, the speed of travel of the pulse along an arterial segment, referred to as the pulse wave velocity, increases. The forward cardiac ejection wave travels through central compliance arteries until it meets forward resistance. The pulse wave is then reflected (reflection wave), where it sums with continuing forward cardiac ejection increasing systolic pressures. Less compliant vasculature returns the reflection wave sooner, making a greater contribution to systolic pressure. Cyclic fatiguing and elastase activity also result in a reduction and fragmentation of vascular elastin (35). Vascular remodeling therefore takes place and results in dilatation and elongation of the aorta and major arteries. These changes are accompanied by impaired endothelial function owing to reduced prostacyclin production by cells which remain. With age, the endothelium undergoes apoptosis, progressive irregularity in cell size and shape, and increased multinucleated giant cells. Endothelial-dependent responses to agonists such as acetylcholine are therefore impaired (36,37). The impaired endothelial function of aging is difficult to separate
from that which results from coexisting hypertension, hypercholesterolemia, and atherosclerosis.
from that which results from coexisting hypertension, hypercholesterolemia, and atherosclerosis.
Age-related changes in the conduction system result from apoptosis and the deposition of collagenous and fatty tissue. Fat accumulates around the sinoatrial node, sometimes producing partial or complete separation of the node from the atrial musculature. There is also a pronounced decrease in the number of pacemaker cells in the sinoatrial node beginning at age 60. At age 75, less than 10% of the cell number found in the young adult remains. Calcification of the atrioventricular node and left and right bundle branches also occur. Thus, older patients often have modest increases in electrocardiographic PR and QT intervals, increased QRS duration and bundle branch blocks, and decreased T-wave amplitude (38). The maximum predicted heart rate (HR) in an octogenarian is 30 beats per minute lower than it was at age 50, and HR in older individuals is also less responsive to β-adrenergic stimulation. In the Framingham cohort, variability in RR intervals declines by 38% between age 40 and 70, reflecting the lesser contribution of autonomic tone to cardiac function with aging (39). Altered autonomic regulation is also demonstrated by reduced heart rate variability (HRV) to head-up tilt testing and impaired baroreflex (40). The Framingham study demonstrated the gradual increase in the prevalence of atrial fibrillation in the population between age 50 and 80 (<0.5%–8.8%) (41). In addition, there is an increase in ambient rate of premature atrial and ventricular contractions as evidenced by holter monitors in healthy adults. Short runs of supraventricular tachycardia occur in up to 33% of healthy individuals over age 60 (42).
Integrated Cardiovascular Performance
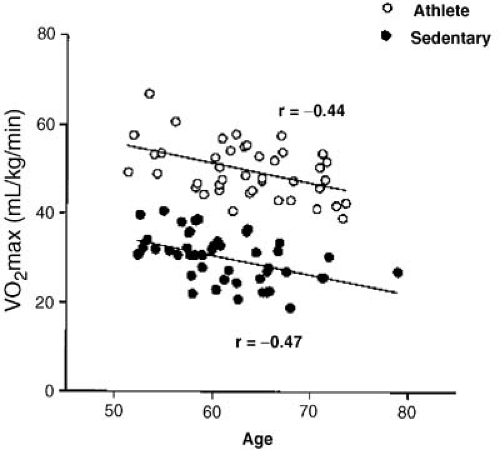
Aerobic capacity (VO2 max) declines with normal aging due to diminished cardiac reserve. Age-related changes in heart function must be differentiated from those resulting from a sedentary lifestyle or other disease processes. Many older patients become inactive, both physically and mentally, which accelerates deterioration and loss of function (43). Early studies found a steady decline in overall cardiovascular performance with aging as judged from exercise training (44). Cardiovascular performance and multiple gated acquisition (MUGA) cardiac volumes were assessed during upright cycle exercise in 40 healthy volunteers (45). Although total exercise duration was similar in young and old, there were age-associated deficits in chronotropic and LV systolic reserve performance. At 10 minutes of exercise in the steady state, older subjects had lower HR and VO2, but higher end-diastolic and end-systolic volume indices than younger subjects. Therefore, maximum cardiac output was preserved because of increased cardiac volumes (preload), despite lower HR, contractility, and greater impedance (afterload) in older subjects. Interestingly, β-blockade in younger individuals also blunts HR response and increases end-diastolic volume with exercise in a similar fashion. This suggests that age-related differences in cardiac performance may be related to a reduction in β-adrenergic responsiveness in the elderly (46). In addition, changes in the periphery, such as decreased muscle mass and increased body fat impair O2 extraction from circulating blood volume in older patients. When VO2 max is normalized for markers of peripheral muscle mass, the proportion of the decline in aerobic capacity attributed to age itself decreased by about 50% (47). More recent work comparing maximum aerobic capacity (VO2 max) in older athletes and sedentary individuals demonstrated that athletes started at higher levels of aerobic capacity, but both groups had similar declines over time (48) (Fig. 33.2). Therefore, there is an inevitable decline in peak performance with age. However, older individuals can derive benefit from successful physical training in the same ways as younger persons in terms of increased exercise tolerance, muscle mass, and improved ventricular performance (49). In fact, motivated fit older persons can also achieve the same peak cardiac output as younger cohorts, albeit by different mechanisms (50).
In summary, the changes in cardiac structure and function with aging certainly have important implications for physiologic responses to exertion as well as clinical responses to disease. Diastolic dysfunction, left ventricular hypertrophy (LVH), and conduction disease may remain below the clinical threshold until coexisting cardiovascular conditions like ischemia, valvular disease, arrhythmia, or hypertension unmask this diminished capacity and trigger symptomatic presentation.
Clinical Pharmacology in the Elderly
Pharmacotherapy is one of the most important interventions in the treatment of elderly patients, but age-related alterations in pharmacokinetics (what the drug does in the patient) and pharmacodynamics (what the drug does to the patient) are important to consider (Table 33.3). Three key components of pharmacokinetics—distribution, metabolism, and excretion—are affected by the aging process. Drug absorption from the gastrointestinal tract does not appear to be affected unless altered by frequently used drugs such as antacids or anticholinergic agents. Once absorbed, however, age-related changes begin to impact drug handling.
First, the liver, which is a major site of oxidative and synthetic drug metabolism, undergoes changes with aging. Autopsy and ultrasound studies have shown a progressive decrease in liver mass after the age of 50. Regional blood flow to the liver at age 65 is reduced by 45% relative to that in a 25-year-old individual. Important changes are also attributed specific hepatic enzyme systems (particularly the cytochrome
P-450 system) (52). These changes are likely responsible for the reduction in hepatic metabolism of drugs that can be as great as 25% over the human lifespan.
P-450 system) (52). These changes are likely responsible for the reduction in hepatic metabolism of drugs that can be as great as 25% over the human lifespan.
TABLE 33.3 Age-Related Pharmacologic Changes in the Elderly | |||
|---|---|---|---|
|
Glomerular filtration rate declines by 40% between age 20 and age 70 owing to diminished renal blood flow and renal mass. One common mistake is overestimating renal function by evaluating the blood urea nitrogen and creatinine in older patients. Because blood urea nitrogen reflects protein ingestion and serum creatinine is produced by the muscle, it is not uncommon for malnourished elderly patients with diminished muscle mass to have normal blood urea nitrogen and creatinine levels even in the presence of significant renal impairment. Even estimated creatinine clearance may not account for reduction in muscle mass component of total body mass, but this remains the best estimate for clinical practice (53). Many standard cardiovascular drugs (low-molecular-weight heparin, GP IIB/IIIA inhibitors, digoxin, diuretics, angiotensin-converting enzyme [ACE] inhibitors, atenolol, nadolol, and clonidine) are affected by renal clearance and adjustments for reduced renal function are recommended. The Cockroft–Gault formula should be applied routinely to estimate renal function in older patients receiving drugs excreted by the kidneys. Changes in creatinine clearance or volume of distribution may prolong half-life and increase drug side effects in the elderly.
Age-related alterations in drug distribution from changes in body composition and plasma proteins have more minor implications. The increase in adipose tissue results in a more extensive distribution and longer half-life of lipid-soluble drugs. The decrease in total body water and reduced volume of distribution leads to higher serum concentrations of water-soluble drugs (54,55). Decreases in serum albumin with age minimally impact drug distribution. However, many drug assays measure the total amount of drug. Because it is the unbound concentration that is pharmacologically active, patients who are hypoalbuminic may actually have unacceptably high drug levels. For example, levels of phenytoin, which is highly bound to albumin, may be misleading interpreting serum levels in the setting of malnourishment or chronic illness.
Drug Use in the Elderly
Drugs can improve function and quality of life in elderly patients, but constant vigilance remains necessary for adverse reactions and interactions. Reduced metabolism and slower elimination increase the propensity for drug complications. Age-related changes in pharmacodynamics further expose the elderly to therapeutic effects and toxicity. Drug use in the elderly cardiovascular patient is also complicated by drug interactions, with many elderly taking as many as 8 to 15 drugs on a daily basis (51). With altered distribution and elimination, diminished reflex and end-organ responses, and exposure to interactions, lower dosages of drugs are intuitive and advisable. Although the ability to distinguish adverse drug effects (e.g., drowsiness, altered cognition, constipation, and falls) from manifestations of disease can be problematic, a basic understanding of pharmacologic principles and simplification of regimens can minimize risks of medication errors and improve compliance.
Heterogeneity of Aging
Data obtained from clinical trial populations may not be generalizable to a heterogeneous elderly population (56). Among older populations with heart disease, large subgroups of individuals also have multiple comorbidities, frailty, or are disabled. One in five (20%) of Medicare beneficiaries have five or more chronic conditions, and 50% take more than five daily medications (57). Similarly, one in five (19.6%) community dwelling elders age 65 years or older depend on others for assistance with activities of daily living (58). In the Women‘s Health Initiative, 16.3% of women over 65 years were frail (59). Frailty is a decline in physiologic reserves associated with an increased vulnerability to adverse outcomes and diminished resistance to stress. Frailty often overlaps with cardiovascular disease, comorbid conditions, depression, and inflammatory dysregulation, all of which may contribute to cardiovascular risk and outcomes (60). In addition to physiologic impairments, the Heart Protection Study (HPS) found that 34% of community-dwelling elderly over age 70 had mild cognitive impairment (61). The Cardiovascular Health Study confirmed a prevalence of mild cognitive impairment in those age 75 years or older of 29% by detailed neuropsychological testing (62). For these vulnerable elderly, face value extension of all practice guidelines may divert attention from the key aspects of care in this population or even result in unintended harm (63). Yet treatment gaps in vulnerable elderly remain a significant problem (64). Patient-specific customization of care is needed to minimize the problem of overtreatment, drug–drug interactions, and medical errors in the vulnerable elderly.
Cardiovascular Risk Factors
The role of conventional cardiovascular risk factors in determining risk in the elderly has been debated. Conventional wisdom held that conventional risk factors such as smoking, hypertension, and dyslipidemia were less important in the aged
because of their diminished relative impact on risk. Yet, although relative risk (RR) associated with a given factor may decline, the higher prevalence of these risk factors, and of cardiovascular disease at older ages, yields greater absolute and attributable risk in the elderly. Therefore, because coronary heart disease (CHD) is the leading cause of death in older individuals, even small reductions in RR may result in a substantial number of lives saved. Chronologic age also stands as a surrogate for unmeasured comorbidity and marks duration of exposure to conventional risk factors. Guidelines still emphasize modification of conventional risk factors regardless of patient age (65).
because of their diminished relative impact on risk. Yet, although relative risk (RR) associated with a given factor may decline, the higher prevalence of these risk factors, and of cardiovascular disease at older ages, yields greater absolute and attributable risk in the elderly. Therefore, because coronary heart disease (CHD) is the leading cause of death in older individuals, even small reductions in RR may result in a substantial number of lives saved. Chronologic age also stands as a surrogate for unmeasured comorbidity and marks duration of exposure to conventional risk factors. Guidelines still emphasize modification of conventional risk factors regardless of patient age (65).
In the elderly, overall risk is best determined by the severity and combination of risk factors. Traditional risk factors (e.g., hypertension, smoking, dyslipidemia, diabetes mellitus) often act as risk multipliers in the setting of end-organ impairment (e.g., LVH, chronic kidney disease). Grids developed from epidemiologic data are available to help estimate long-term risks in specific patients (10-year risk), but are quite limited past age 75 (66). In elderly populations, the overall risk must include consideration of subclinical cardiovascular disease as well as existing end-organ impairments. Indicators of subclinical disease include peripheral arterial bruits, electrocardiographic (ECG) abnormalities, low ankle–brachial indices, or atherosclerosis on carotid ultrasound. Newer risk factors are also emerging which reflect declining reserves in organ function (e.g., creatinine clearance, pulse pressure, LVH, and anemia).
Hypertension
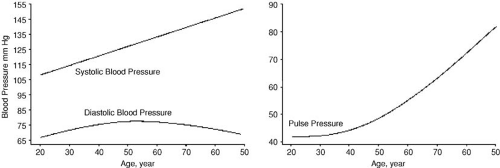
Morphologic changes in the aorta and the peripheral vessels make systolic hypertension an inevitable consequence of aging (67,68). Population trends demonstrate increased systolic blood pressure and decreased diastolic blood pressure with aging (Fig. 33.3). Hypertension, defined as systolic pressure above 140 mm Hg, alone or in association with diastolic pressure above 90 mm Hg, occurs in more than 50% of the elderly population (69). Isolated systolic hypertension affects 30% of adults age 80 years or older (70). Thus, hypertension is a hallmark of aging, but is also a modifiable cardiovascular risk factor in the elderly.
Multiple studies have assessed the risks of elevated systolic blood pressure in the elderly (71,72,73). In the Framingham study, participants aged 65 to 94 years with a systolic pressure higher than 180 mm Hg had a three- to fourfold increased risk of CHD compared to patients with a systolic pressure lower than 120 mm Hg (67). Wide pulse pressure has been identified as a marker for arterial stiffness and important risk indicator for cardiovascular complications in the elderly (74,75,76). The prognostic importance of wide pulse pressure is greater than either systolic blood pressure or diastolic blood pressure. The Framingham Heart Study found that the risk of cardiovascular events increased by 16% per 10–mm Hg rise in systolic blood pressure, but by 23% per 10–mm Hg rise in pulse pressure (77). After adjusting for demographics, morbidity, and risk factors, each 10–mm Hg increase in pulse pressure was associated with a 12% increase in CHD death and a 14% increase in CHF. The National Health and Nutrition Examination Survey (NHANES) and pooled European and Chinese Trials confirmed the positive associations between pulse pressure and MI, stroke, cardiovascular mortality, and all-cause mortality (78,79,80). Treatment of hypertension reduces the rate of stroke, CHF, chronic renal failure, and CHD as well as all-cause mortality (81,82).
Nonpharmacologic treatment should be pursued in all hypertensive patients with salt restriction, exercise, abstinence from alcohol, and weight reduction when appropriate. The Trial of Nonpharmacologic Interventions in the Elderly (TONE) enrolled 975 hypertensive patients between the ages of 60 and 80. Patients were randomly assigned to undergo a reduction in dietary sodium, weight reduction, both, or neither. Each intervention significantly reduced blood pressure, but the combination was most successful (83).
Antihypertensive medication is justified if systolic pressures are 160 mm Hg or higher, and between 140 and 159 mm Hg based on individual risk assessments (84,85). A greater benefit from the treatment of systolic hypertension is also seen among those with wide pulse pressure. The number of hypertensives needed to treat to prevent one cardiovascular death was 119 when pretreatment pulse pressure were between 65 and 89 mm Hg, and just 63 when pretreatment pulse pressure were greater than 90 mm Hg (86). The Systolic Hypertension in the Elderly Program (SHEP) was the first randomized, controlled trial of the treatment of isolated systolic hypertension (87). Nearly 450,000 patients older than age 60 were screened, and 4,736 were randomly assigned to treatment (stepwise chlorthalidone or atenolol) or placebo and followed for approximately 5 years.
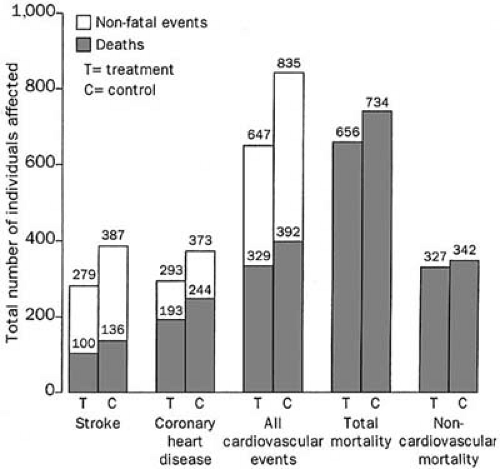
Baseline systolic blood pressure ranged from 160 to 219 mm Hg and diastolic blood pressure were less than 90 mm Hg. Active treatment lowered the risk of stroke (36% risk reduction) and MI (28% risk reduction) and improved survival. A recent metaanalysis combining eight trials of systolic hypertension in the elderly further confirms the enduring benefit from treatment (88). In this metaanalysis, treatment lowered systolic blood pressure by an average of 10 mm Hg and diastolic blood pressure by 4 mm Hg. In so doing, the treatment group had a lower risk of stroke (30% risk reduction), cardiovascular events (23% risk reduction), and mortality (13% risk reduction) (Fig. 33.4). Greatest treatment benefit was again seen in subgroups including men, those aged 70 years or older, with wider pulse pressure, or prior cardiovascular complications. In a subgroup analysis of 1,670 subjects over 80 years of age, antihypertensive therapy reduced stroke by 34% and heart failure by 39%, although there was a nonsignificant 6% increase in mortality (89). The Hypertension in the Very Elderly Trial (HYVET) will enroll 2,100 patients older than 80 years of age and will compare two groups randomized to indapamide with or without perindopril for a primary end point of stroke at 5 years. This study should answer lingering questions about whether active antihypertensive therapy is associated with significant reductions in cardiovascular morbidity and mortality in the very elderly age group as it clearly is in young elderly (90).
Baseline systolic blood pressure ranged from 160 to 219 mm Hg and diastolic blood pressure were less than 90 mm Hg. Active treatment lowered the risk of stroke (36% risk reduction) and MI (28% risk reduction) and improved survival. A recent metaanalysis combining eight trials of systolic hypertension in the elderly further confirms the enduring benefit from treatment (88). In this metaanalysis, treatment lowered systolic blood pressure by an average of 10 mm Hg and diastolic blood pressure by 4 mm Hg. In so doing, the treatment group had a lower risk of stroke (30% risk reduction), cardiovascular events (23% risk reduction), and mortality (13% risk reduction) (Fig. 33.4). Greatest treatment benefit was again seen in subgroups including men, those aged 70 years or older, with wider pulse pressure, or prior cardiovascular complications. In a subgroup analysis of 1,670 subjects over 80 years of age, antihypertensive therapy reduced stroke by 34% and heart failure by 39%, although there was a nonsignificant 6% increase in mortality (89). The Hypertension in the Very Elderly Trial (HYVET) will enroll 2,100 patients older than 80 years of age and will compare two groups randomized to indapamide with or without perindopril for a primary end point of stroke at 5 years. This study should answer lingering questions about whether active antihypertensive therapy is associated with significant reductions in cardiovascular morbidity and mortality in the very elderly age group as it clearly is in young elderly (90).
Pharmacologic treatment in older persons follows the same principles outlined for the general care of hypertension in JNC VII (91). The choice of agents in elderly hypertensives is as broad as in younger patients, although the best studied drugs are diuretics and β-blockers. The large National Institutes of Health–funded Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT) compared treatment with a diuretic, calcium channel blocker, and ACE inhibitor across 33,357 participants over 5 years of follow up. There were no differences in mortality between groups, but patients treated with thiazide diuretics achieved a lower systolic blood pressure, and demonstrated advantages in rates of CHF, stroke, and combined cardiovascular events (92). Interestingly, a subanalysis of losartan versus atenolol in the elderly found that angiotensin receptor blockers resulted in better end point reduction in the subgroup with high pulse pressures (93). Certain agents may also be selected for elderly with coexisting conditions. For example, ACE inhibitors may be preferred for elderly diabetics, or those with renal disease or heart failure because of their favorable effects on neurohumoral state. In addition, drug intolerances often dictate selection of agents among available choices. In summary, the evidence that systolic hypertension and pulse pressure are risk factors for cardiovascular and cerebrovascular complications in the elderly is clear, and evidence that treatment lowers risk is robust.
Dyslipidemia
The importance of dyslipidemia as a risk factor in the elderly has been debated. This relates, in part, to epidemiologic studies looking at hypercholesterolemia as a risk factor in the elderly that were confounded by older people with coexisting noncardiac illness and frailty who had low cholesterol levels (94,95). Perhaps the best evidence linking cholesterol and risk in the elderly comes from the Rotterdam study, a population-based study of 6,006 individuals older than 55 years (96). Men and women age 70 years or older with total cholesterol in the top quartile had a greater risk of MI compared with those in the lowest quartile, RR 3.2 (95% confidence interval [CI], 1.3–7.7) and RR 2.9 (95% CI, 1.3–6.6), respectively. Likewise, men and women age 70 years or older with high-density lipoprotein in the top quartile had a lower risk of MI compared with those in the lowest quartile, RR 0.5 (95% CI, 0.3–0.9) and RR 0.4 (95% CI, 0.2–0.9), respectively. The findings in this study have been confirmed by other studies and evidence now supports that dyslipidemia continues to be a risk factor in the elderly, although data past age 75 are sparse.
Evidence for the treatment for dyslipidemia in the elderly is stronger for secondary prevention. The large secondary prevention trials, Cholesterol and Recurrent Events Trial (CARE) and Long-term Intervention with Pravastatin in Ischemic Disease (LIPID), excluded patients older than 75 years, and the Scandinavian Simvastatin Survival Study Group trial (45) set an upper age limit of 69 years (97,98,99). Trials confirmed the benefit of statins for secondary prevention in young elderly (100,101). However, two recent studies have added to the evidence base in the elderly. The Heart Protection Study (HPS), which compared simavastatin to placebo in high-risk secondary prevention, enrolled patients up to age 80, and specifically targeted an older population (52% age ≥65 years) (102). In this study, the 5,806 patients aged 70 years or more had the same absolute risk reduction with simvastatin as was demonstrated in patients younger than age 65 years (5.1% versus 5.2%). This resulted in a number needed to treat in the oldest subgroup of 20 patients to prevent one cardiovascular death or MI. The Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER) trial was performed exclusively in the elderly. In this trial, 5,804 high-risk individuals age 70 years or older (range, 70–82; mean, 75) were randomized to pravastatin or placebo (103). Pravastatin treatment was associated with a 15% relative and a 2.1% absolute risk reduction compared with placebo at 3.2 years of follow up. The magnitude of risk reduction was less than anticipated, but could not be compared to a younger subgroup. Observational studies also show that those elderly with heart disease treated with statins do better than those without statin therapy. The Intermountain Heart Collaborative Study followed a cohort of 7,220 individuals with coronary artery disease by statin treatment over a mean
follow-up duration of 3.3 years (104). Elderly patients were less likely to receive statins, but mortality was similarly decreased with statin use across all age groups. For those age 80 years or more, mortality was 29.5% among statin nonusers and 8.5% among statin users (hazard ratio 0.5, P < .036). In addition, these studies have shown the tolerability of lipid lowering medications in the elderly to be similar to younger patients.
follow-up duration of 3.3 years (104). Elderly patients were less likely to receive statins, but mortality was similarly decreased with statin use across all age groups. For those age 80 years or more, mortality was 29.5% among statin nonusers and 8.5% among statin users (hazard ratio 0.5, P < .036). In addition, these studies have shown the tolerability of lipid lowering medications in the elderly to be similar to younger patients.
Many primary prevention trials do not permit meaningful elderly subgroup analyses. The oldest patient in West of Scotland Coronary Prevention Study (WOSCOPS) was age 65, and the oldest patient in AFCAPS/TexCAPS was 73 (105,106). The Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), which enrolled patients up to age 79 years, found that primary prevention treatment with atorvastatin significantly reduced the risk of cardiovascular death and nonfatal MI in patients older than 60 years (hazard ratio 0.64, 0.47–0.86), but only trended to reduction in those age 60 years or younger (hazard ratio 0.66, 0.41–1.06) (107). With these data, the easiest group in which to pursue primary prevention is the young old, namely, those between 65 and 75 years of age. High-risk elderly patients with substantial remaining life-years are likely to benefit from primary prevention, but definitive data are yet to be obtained.
Diabetes Mellitus
Diabetes and metabolic syndrome are powerful predictors of primary and secondary cardiovascular events in patients of all ages. Total body fat and visceral adiposity increase until age 65, and are often accompanied by insulin resistance and diabetes (108). The elderly are also susceptible to glucose intolerance owing to diminished reserves in the mechanisms to lower glucose, and susceptible to hypoglycemia because of lower levels of regulatory hormones that raise glucose, like glucagon and growth hormone (109). NHANES III determined that metabolic syndrome is prevalent in approximately 44% of the general U.S. population older than 50 years (110). Metabolic syndrome is associated with an increase in incidence of cardiovascular disease and future cardiovascular events over four years after accounting for other factors (odds ratio [OR] 1.38; 95% CI 1.07–1.79). The metabolic syndrome has also been shown to be an independent predictor of arterial stiffness in the Baltimore Longitudinal Study of Aging (111).
Type 2 diabetes is less common in older persons, occurring in approximately 15% of the general population older than age 70, with a peak incidence at age 75 to 84 in men (16.5%) and women (12.8%) (112). However, in a population with ACS, the prevalence of diabetes is higher, and peaks 10 years earlier (29.6% age <65; 38.8% ages 65–74; 35.5% ages 75–84; and 24.7% age ≥85) (113). Insulin resistance rather than insulin deficiency is the hallmark of diabetes in older age (114,115). Risk stratification is of particular importance in older diabetics because of the variability in their outcomes related to other risk factors, as well as the common occurrence of unrecognized MI (108). Although randomized trials for diabetic treatment were conducted in younger cohorts, abundant epidemiologic evidence suggests glucose control is equally beneficial in the elderly (116,117).
Smoking
The prevalence of smoking dramatically decreases with age. For example, almost half of patients with ACS under age 65 are smokers (46.6%), compared with just 4% of patients age 85 or older (113). Similarly, smoking is a stronger relative risk factor for cardiovascular events in the young (118). Smoking cessation post-MI is shown to reduce mortality by 25% to 50%, most of which occurs in the first year (119). Thus, the few smokers who continue to smoke in their later years will still benefit from smoking cessation.
Life Style
Diet, exercise, and psychological well-being determine the pace of aging as well as the quality of life in later years. Dietary intake in the elderly should mirror that recommended for health, yet total caloric needs decline over time. In fact, the only lifestyle intervention proven to lengthen life (albeit in animals) is caloric restriction by 30% (120). This level of dietary restriction would be poorly tolerated in humans, but emphasizes the role of dietary intake on metabolic activity (insulin secretion, body temperature) and other process central to aging and disease. However, the epidemiologic data between diet and cardiovascular outcomes are not strong. The Cardiovascular Health Study was able to document a weak relationship between healthier dietary patterns (low fat, low cholesterol) and longer 10-year survival (121).
Exercise has tremendous value in reducing the risk of CHD mortality and morbidity and slowing the decline in physical function. The Honolulu Heart Program, which studied 2,678 physically capable elderly men aged 71 to 93, demonstrated a relationship between lower risk of CHD and longer distance walked (122). Men who walked more than 1.5 miles per day had half the risk of CHD as those who walked less than 0.25 miles a day (2.5% versus 5.1%). Combined with the evidence that an active life style reduces the risk of CHD in younger individuals, these data suggests that walking has disease prevention value for the elderly.
Social isolation and depression are also common in elderly patients, and are independently associated with recurrent events after MI and death from cardiovascular disease (123,124). Emerging evidence indicates that treatment of these factors also improves outcomes; however, clinical trials are difficult to control in the psychosocial area.
Left Ventricular Hypertrophy
A poorly appreciated risk factor in the elderly is LVH, which is independently associated with higher risk of cardiovascular events (125). With age, development of LVH occurs in parallel to arterial stiffening, and is accelerated by the presence of hypertension and obesity. Because hypertension and obesity also increase with age, LVH may be present in over 50% of people older than 65 years. Some antihypertensive agents can slow the progression or reverse the course of LVH (126,127). The Losartan Intervention for Endpoint Reduction trial randomized 1,326 patients with isolated systolic hypertension and ECG–LVH to losartan or atenolol with hydrochlorothiazide as a second agent for 4.5 years of follow up (128). In the subpopulation who had LVH at entry, the degree of LVH was associated with cardiovascular morbidity and mortality, independent of blood pressure lowering or treatment arm (129). In a follow-up analysis, for each standard deviation decrease in LV mass index, there was a 22% reduction in the composite cardiovascular end point predicted by blood pressure–lowering effect. Treatment with losartan decreased ECG-LVH to a greater extent than atenolol (130).
Chronic Kidney Disease
Abnormal renal function is a significant predictor of all-cause and cardiovascular mortality, cardiovascular disease, claudication, and CHF in elderly individuals. The National Kidney Foundation KDOQI Guidelines have named five stages of
chronic kidney disease (CKD) (131). Risk for adverse outcomes increases notably in stage III and beyond (creatinine clearance <60 cc/min). In the Cardiovascular Health study, 11.2% of participants had an elevated serum creatinine level (≥1.3 mg/dL for women or ≥1.5 mg/dL for men) (132). In a large epidemiologic study, CKD stage III or greater was present in 7.4% of the population. Individuals with CKD were more likely to reach a composite outcome of MI, stroke, or death over 10 years of follow up than were those without CKD (30.1% versus 13.2%, respectively) (133). CKD also predicts a higher risk of mortality and bleeding following acute MI (134,135). Patients with CKD have a greater degree of subclinical cardiovascular disease, metabolic derangements, and longer exposure to risk factors (136). Patients with CKD have also been shown to have small LDL particles, hyperfibrinoginemia, homocysteinemia, increased inflammatory markers, and are often anemic (137,138,139).
chronic kidney disease (CKD) (131). Risk for adverse outcomes increases notably in stage III and beyond (creatinine clearance <60 cc/min). In the Cardiovascular Health study, 11.2% of participants had an elevated serum creatinine level (≥1.3 mg/dL for women or ≥1.5 mg/dL for men) (132). In a large epidemiologic study, CKD stage III or greater was present in 7.4% of the population. Individuals with CKD were more likely to reach a composite outcome of MI, stroke, or death over 10 years of follow up than were those without CKD (30.1% versus 13.2%, respectively) (133). CKD also predicts a higher risk of mortality and bleeding following acute MI (134,135). Patients with CKD have a greater degree of subclinical cardiovascular disease, metabolic derangements, and longer exposure to risk factors (136). Patients with CKD have also been shown to have small LDL particles, hyperfibrinoginemia, homocysteinemia, increased inflammatory markers, and are often anemic (137,138,139).
Coronary Heart Disease
The incidence of cardiovascular disease, peripheral arterial disease, stroke, and CHF all increase with age. Although not inevitable, atherosclerosis is often present and increasingly severe in the elderly. At autopsy, more than 50% of people older than the age of 50 were found to have significant stenosis of at least one coronary artery. The severity and number of stenoses increase with each age decile (140). The relationship between gender and risk for cardiovascular disease reverses past age 65. Although cardiovascular disease has a greater prevalence in men prior to this age, its prevalence in women exceeds that in men past this age (Fig. 33.5).
The classic description of symptomatic coronary artery disease is from the perspective of young white men, yet more than half of acute MIs occur past age 65 years and in women. In these groups, chest pain diminishes in favor of other ischemic symptoms, particularly dyspnea, dizziness, and fatigue. Coexisting inactivity or comorbid illness further delay recognition of ischemic symptoms or modify its character. The ECG of an elderly patient may show nonspecific changes or intraventricular conduction delays at baseline, making interpretation in the acute setting more challenging. With unclear symptoms and nondiagnostic ECGs, diagnostic testing is often the next step. When the diagnosis of CHD remains questionable, angiography should be considered. However, because atherosclerosis is apt to be present in the absence of symptomatic CHD, the coronary arteriogram may provide a false-positive result in reference to symptoms, even though it may establish a cardiac diagnosis.
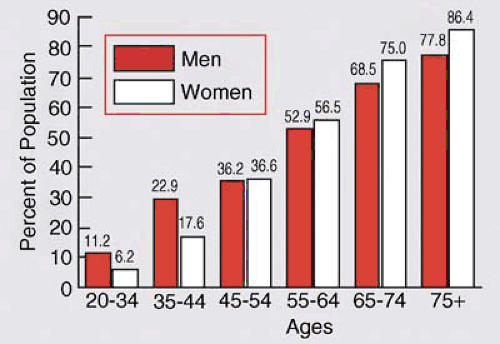 FIGURE 33.5. Prevalence of cardiovascular diseases in Americans age 20 or older by age and gender. From NHANES: 1999–2002. (Source: From AHA statistical update 2004. Available: http://www.amheart.org. Accessed August 18, 2005.) |
Stable Angina
Stable angina is reported in 10% of people older than age 65. The diagnosis of ischemic heart disease becomes increasingly difficult as symptoms are atypical, vague, or silent. Of the 5,888 participants in the Cardiovascular Health Study, 15.3% evidenced a prior MI, and 201 (22.3%) of these were clinically unrecognized. Predictors of unrecognized or “silent” MIs include female gender, older age, absence of preceding angina, and self-assessed health as good or excellent (141). Prognosis of patients with unrecognized MIs is the same if not higher than those with recognized MIs. Therefore, detailed information on functional capacity and recent changes in activities of daily living are of importance in identifying symptoms and directing evaluation.
Noninvasive Testing
In the elderly, symptoms are the key factor in the decision to pursue testing. Exercise ECG testing is the simplest and least expensive approach (142,143). However, exercise protocols may need to be modified with regard to speed and incline. Although HR may not reach 85% predicted, increases in systolic blood pressure frequently occur, and therefore adequate HR–blood pressure product may still be achieved. The high incidence of resting ECG abnormalities in this age group lowers the sensitivity and specificity of the ECG portion of the study; however, duration of exercise is more important than ST-segment depression (144,145). Nuclear and echocardiographic imaging are often added to increase diagnostic yield. Older patients may have musculoskeletal or balance problems that limit their ability to engage in full treadmill exercise, in which case pharmacologic stress tests (dipyridamole, adenosine, and dobutamine hydrochloride) are performed.
Coronary Arteriography
Coronary arteriography is the standard for establishing the presence and extent of coronary atherosclerosis. The decision to use coronary arteriography is complicated by the high prevalence of significant coronary atherosclerosis, which makes attribution of functional or symptomatic significance challenging. In light of its limited diagnostic clarity in the elderly, angiography should primarily be undertaken to assess the suitability of coronary anatomy for revascularization. The complication rate for arteriography is not much increased in the elderly, provided that the patient is stable and has no major comorbidity that would alter their risk (146).
Revascularization
Because of the extent of disease, symptoms in the elderly may be refractory to medical therapy. Furthermore, the elderly patient may be intolerant of the dose increases in medication necessary for symptom relief. Therefore, mechanical revascularization is an attractive option when symptoms persist and limit function or quality of life. Randomized clinical trials comparing revascularization strategies have included few elderly patients, but analyses of retrospective studies provide descriptive information (147,148,149,150,151). Morbidity and mortality from procedures in the elderly depends greatly on case selection. In a large registry of nearly 110,000 patients, the 7,472 octogenarians
undergoing coronary artery bypass grafting (CABG) had an in-hospital mortality of 3.8% compared to 1.1% in the younger patients. They also had a slightly higher rate of Q-wave MI, stroke, renal failure, and vascular complications (152). In this study, however, the mortality for octogenarians varied 10-fold depending on the presence of comorbidities (0.8%–7.2%). In a study of the Medicare database, 24,461 octogenarians who underwent isolated CAGB had a 30-day mortality of 10.5% compared to 4.3% in the cohort 65 to 80 years old (153). Another registry of 22 centers and 64,467 patients (4,306 of whom were older than 80 years), found that mortality following isolated CABG was 8.1% among octogenarians and 3% in younger patients (154). In addition to having advanced atherosclerosis, the octogenarians also had more comorbid conditions, including peripheral vascular disease, pulmonary disease, and renal disease, yet among those octogenarians without associated comorbidity, mortality following CABG was just 4.2%. However, aortic valve replacement (10.1%) and mitral valve replacement (19.6%) performed with CABG markedly increased in-hospital mortality for octogenarians. A recent analysis pooled the observational outcomes of patients aged 75 years or older who underwent revascularization with either percutaneous coronary intervention (PCI; n = 48,439) or CABG (n = 180,709) between 1990 and 1999. During this interval, the proportion of patients aged 75 years or older undergoing revascularization rose by 10%. Composite estimates for in-hospital mortality following PCI was 3.0% (range 1.5%–5.2%), and following CABG was 5.9% (range 4.9%–8.4%). Age remained a major determinant of procedural risk, along with procedural urgency, LV dysfunction, and prior CABG (155).
undergoing coronary artery bypass grafting (CABG) had an in-hospital mortality of 3.8% compared to 1.1% in the younger patients. They also had a slightly higher rate of Q-wave MI, stroke, renal failure, and vascular complications (152). In this study, however, the mortality for octogenarians varied 10-fold depending on the presence of comorbidities (0.8%–7.2%). In a study of the Medicare database, 24,461 octogenarians who underwent isolated CAGB had a 30-day mortality of 10.5% compared to 4.3% in the cohort 65 to 80 years old (153). Another registry of 22 centers and 64,467 patients (4,306 of whom were older than 80 years), found that mortality following isolated CABG was 8.1% among octogenarians and 3% in younger patients (154). In addition to having advanced atherosclerosis, the octogenarians also had more comorbid conditions, including peripheral vascular disease, pulmonary disease, and renal disease, yet among those octogenarians without associated comorbidity, mortality following CABG was just 4.2%. However, aortic valve replacement (10.1%) and mitral valve replacement (19.6%) performed with CABG markedly increased in-hospital mortality for octogenarians. A recent analysis pooled the observational outcomes of patients aged 75 years or older who underwent revascularization with either percutaneous coronary intervention (PCI; n = 48,439) or CABG (n = 180,709) between 1990 and 1999. During this interval, the proportion of patients aged 75 years or older undergoing revascularization rose by 10%. Composite estimates for in-hospital mortality following PCI was 3.0% (range 1.5%–5.2%), and following CABG was 5.9% (range 4.9%–8.4%). Age remained a major determinant of procedural risk, along with procedural urgency, LV dysfunction, and prior CABG (155).
The heterogeneity of the aging process and competing risks makes assessment of the total patient, as well as the extent of disease, key to successful decision making and outcomes (156). Although better case selection and technical advances will undoubtedly improve mortality and morbidity, substantially higher risks will certainly persist in the elderly. It is also fair to say that the key determinant of short- and long-term outcomes is preexisting functional status, more than the extent of coronary artery disease (157,158).
Acute Coronary Syndromes
Unstable Angina and Non–ST-Segment Elevation Myocardial Infarction
Non–ST-segment elevation MI is the most common form of myocardial infarction in the elderly, accounting for 55% of MIs in patients above age 85 but less than 40% of MIs in patients below age 65 (159). The higher proportion non–ST-segment elevation MIs in the elderly has been attributed to the higher prevalence of prior MIs, multivessel disease, hypertension, and ventricular hypertrophy, all of which contribute to increased subendocardial ischemia. With progressively older age, patients with ACS are more likely to be female; from 30% below age 65 to 62% over age 85 years (113). In addition, traditional risk factors (e.g., hypertension, diabetes, smoking, hyperlipidemia) diminish in the oldest groups compared to younger counterparts, whereas comorbidities (e.g., CHF, stroke, renal dysfunction) become increasingly common (113).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree