Asthma |
Asthma is characterized by recurrent attacks of breathlessness and wheezing, which vary in severity and frequency from person to person.1 More than 18 million adults and 7 million children are affected in the United States alone, making asthma one of the most common chronic diseases in this country.2 Attempts to elucidate the underlying pathophysiology of the disease have led to the realization that asthma truly is a protean disease with various cell types and mechanisms playing variable but important roles in each patient. This degree of mechanistic variation explains the numerous phenotypes of this disease as well as the differences in response to treatment.
By the simplest definition, the pathogenesis of asthma involves bronchoconstriction, airway inflammation, and airway hyperresponsiveness.3 It is, however, the complex interplay between these factors that defines the disease in general and specifically in an individual patient. A closer examination of the factors involved in each of these components allows a better understanding of this complex disease.
THE ACUTE INFLAMMATORY RESPONSE IN ASTHMA
Perhaps the best illustration of the features of the acute inflammatory response, that is central to the pathogenesis of asthma, is the reaction to the initial and then subsequent exposures to inhaled antigen. While important cellular and molecular mediators will be described later in greater detail, a brief review of the acute inflammatory response serves as a foundation upon which further concepts can be introduced to illustrate the variable but persistent changes that occur in the airway in asthma. When a novel antigen is introduced to the airway of an at-risk individual, it initially becomes trapped in the mucus lining the airway. Here it can be taken up by antigen presenting cells, most notably dendritic cells, which are distributed through the epithelium of the airways.4,5 After the uptake of allergen, the dendritic cells travel to pulmonary lymph nodes whereby the antigen is presented to naïve CD4+ T cells.5 Signals derived from the dendritic cell determine which type of CD4+ T cell will be produced. Prior to this event, the dendritic cell is influenced by a complex network of molecular signals that are derived from airway epithelial cells and other local cell types. In allergic inflammation, for example, thymic stromal lymphopoietin (TSLP) and granulocyte–monocyte colony stimulating factor (GM-CSF), which are derived from bronchial epithelial cells, and induce the dendritic cell to promote TH2 differentiation of naïve CD4+ T cells, thus setting up an environment favorable to the eventual development of allergic inflammation.5 Upon rechallenge with the sensitizing antigen, these now TH2 differentiated CD4+ T cells are recruited back to the airway by other signals, such as the chemokines CCL17 and CCL22, secreted by dendritic cells.6 Upon arrival in the airway, the CD4+ TH2 cells become key sources of the TH2 cytokines, namely IL-4, IL-5, and IL-13, which serve as the molecular catalysts to establish a framework for acute allergic inflammation.6
After rechallenge with antigen, the local environment of the airways is now rich with TH2 cytokines, which act on other cell types, either present or recruited to the airway, to propagate the acute allergic inflammatory response. B cells, in the presence of IL-4 and IL-13, are influenced to produce antigen-specific IgE, which binds to high-affinity IgE receptors (FcεRI) on mast cells (MC). When inhaled antigen cross-links the membrane-bound IgE on the mast cells, a variety of preformed and synthesized meditators are released to cause bronchoconstriction, airway edema, and local tissue damage.7 Mast cells also release chemoattractants such as leukotrienes and cytokines, to recruit a variety of other cells, including eosinophils, basophils, neutrophils, and lymphocytes, which then contribute to the late phase inflammatory response.7 The eosinophil appears to be, in most cases, the most important and abundant inflammatory cell associated with the late phase response and possibly contributes to the subsequent airflow obstruction.8 The vast numbers of mediators the eosinophil produces are reviewed later in the chapter. Eosinophil products can cause local tissue damage, mucus hypersecretion, increased vascular permeability, smooth muscle contraction, and a sustained inflammatory response whereby other cell types are recruited to the site of inflammation to perpetuate the reaction.7,8 The roles of neutrophils and basophils in the pathogenesis of the acute and late-phase allergic inflammatory responses are less well defined.
While the acute allergic response to allergen illustrates the pattern of inflammation seen in asthma, it should be noted that other forms of inflammation can and do play important roles in asthma. Viral respiratory infections, especially with human rhinovirus (HRV), are important triggers for asthma exacerbations.9 The response to HRV is a primarily TH1 driven response with increased production of IL-8 and IL-1β and the appearance of airway neutrophilia, as opposed to the strong TH2 response seen after allergen exposure.9,10 In asthma, there is evidence that diminished production of the type I and III interferons, antiviral cytokines, may be deficient in some patients with asthma thus leading to increased risk for viral respiratory infections and a greater susceptibility to exacerbations of asthma.9,10
This pattern of acute- and late-phase inflammatory response to antigen is, however, a central component of asthma. Chronic inflammation is a later development of the disease and will be described later. Many of the cell types and inflammatory mediators seen in asthma were briefly touched upon in the preceding paragraphs and will now be further developed.
CELL TYPES IN ASTHMA
A study of the pathogenesis of any complex disease begins at the cellular level. The importance of cells of the immune system, including mast cells, basophils, CD4+ T cells, eosinophils, neutrophils, macrophages, dendritic cells, and T lymphocytes, as well as their molecular mediators has long been recognized and appreciated in the development and regulation of inflammation. The contribution of airway smooth muscle cells, especially in relationship to the acute asthmatic response, has also been well documented. More recently, epithelial cells of the airway have become the focus of intense research and emerging importance to both acute and chronic inflammation in asthma. Their role in airway inflammation, and especially in the airway remodeling as is seen in the chronic forms of asthma, is being increasingly cited as a major contributor to the severity of this disease.
 CELLS OF THE IMMUNE SYSTEM IN ASTHMA
CELLS OF THE IMMUNE SYSTEM IN ASTHMA
The contributions of each of the aforementioned immune system cell types in the pathogenesis of asthma are considered below.
Mast Cells
Human mast cells are derived from the same CD34+/cKit+ hematopoietic stem cell population that also gives rises to eosinophils, basophils, neutrophils, and monocytes.11 They are resident cells in most tissues of the body and are commonly found in association with blood vessels, nerves, and surfaces that have contact with the external environment.12 Mast cells exist in two types in humans and are differentiated by their immunohistochemical staining properties.13 MCT mast cells contain only the neutral protease tryptase, while MCTC mast cells contain chymase, carboxypeptidase A3, and cathepsin G-like protease in addition to tryptase.13–15 In normal lung tissue, mast cells are located in the subepithelium of the bronchi, bronchioles, and alveolar walls and are almost exclusively of the MCT type.13 This distribution of mast cell types is also seen in mild asthma. In severe asthma, however, mast cells in the submucosa are decreased in number and are primarily of the MCTC type.16 MCTC mast cells are also seen in the airway epithelium of severe asthma, a finding not seen in normal lungs or in milder disease.16 Mast cells increasingly infiltrate airway smooth muscle bundles in asthma where they likely contribute to ongoing bronchoconstriction through release of their mediators.17
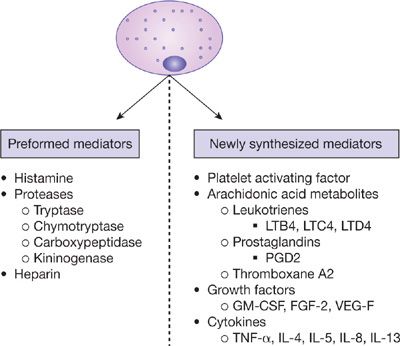
While mast cells appear to have some importance in nonallergic asthma as well, they are essential components of the allergic (IgE-mediated) response seen in many asthma patients.18 Antigen-specific IgE molecules bind allergen and cross-link high-affinity IgE receptors (FcεRI) present on the mast cell surface. This results in the release of preformed mediators, such as histamine, tryptase, chymase, and heparin, as well as tumor necrosis factor-alpha (TNF-α) and vascular endothelial growth factor (VEGF) in some cases (Fig. 44-1).19 Upon activation, mast cells also generate and release newly synthesized mediators, which contribute to the ongoing inflammatory milieu. These include leukotrienes (predominantly LTC4), prostaglandins (predominantly PGD2), thromboxane A2, platelet activating factor (PAF), growth factors including GM-CSF, fibroblast growth factor-2, and VEGF, and various other cytokines including TNF-α, IL-4, IL-5, IL-8, and IL-13 (Fig. 44-1).19,20
Figure 44-1 The mast cell and its mediators. GM-CSF, granulocyte-monocyte colony stimulating factor; FGF-2, fibroblast growth factor 2; VEGF, vascular endothelial growth factor; TNF-α, tumor necrosis factor alpha.
The effect of mast cell mediator release contributes to numerous features in the asthmatic response.7 Histamine, leukotrienes, and the various proteases increase mucus production. Prostaglandins, leukotrienes, thromboxane A2, and histamine cause bronchoconstriction and increase vascular permeability. The various proteases cause local tissue damage and are important in the activation of various protein precursors. Finally, synthesized cytokines contribute to the recruitment, differentiation, and activation of other inflammatory cells, resulting in the propagation of the inflammatory response.
Basophils
In addition to sharing a common progenitor cell, basophils share many similarities with mast cells, with the exception that basophils are present primarily in the peripheral circulation. Both cells express FcεRI on their cell surface and release both preformed, as well as newly synthesized mediators and cytokines, upon cross-linking by IgE–antigen complexes. The major preformed mediator released from basophils is histamine. Preformed heparin and tryptase are also released, albeit at lower concentrations than mast cells.21 Basophils synthesize and release LTC4 upon activation, but unlike mast cells, they do not produce PGD2.21 Upon activation, basophils produce large quantities of IL-4 and IL-13, cytokines that play an important role in the TH2 differentiation, which will be discussed later.21 More recently, two other roles of basophils in the pathogenesis of asthma have been discovered, both of which also play important roles in TH2 differentiation. First, basophils can act as antigen presenting cells via their expression of major histocompatibility complex (MHC) class II and co-stimulatory molecules.22 Second, basophils, along with eosinophils, are also the primary target of IL-33, a potent promoter of allergic inflammation and TH2 polarization.23
Eosinophils
Eosinophils, like basophils and mast cells, are granulocytes derived from CD34+ hematopoietic stem cells. Early eosinophil production is highly dependent upon the presence of GM-CSF and IL-3.24 Eosinophil precursors are recruited to the airway in asthma as the result of cytokine and chemokine signaling, which involves IL-5, eotaxins, RANTES, macrophage inflammatory protein (MIP)-1α, and macrophage chemotactic factors 2,3, and 4 (MCP-2,3,4).25 IL-5 is critically important for the terminal differentiation of eosinophils and release from the bone marrow.24
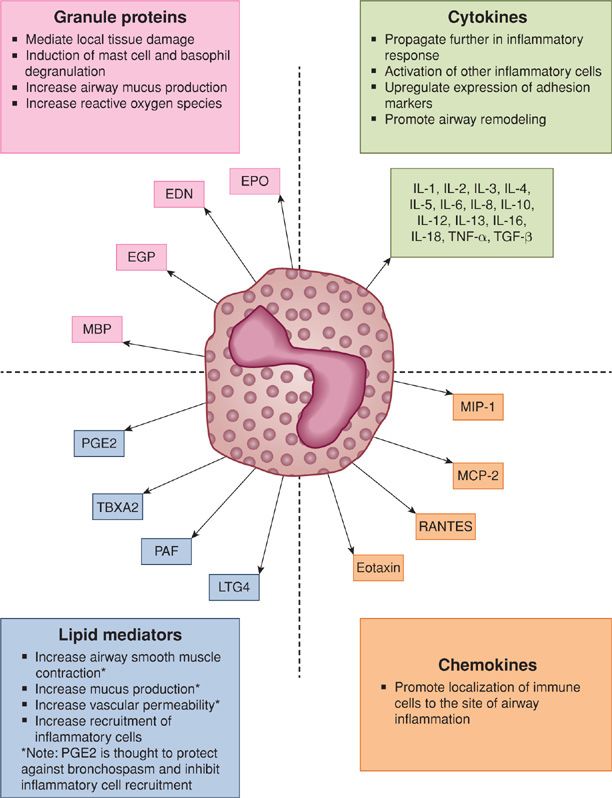
Once in the airway, eosinophils are activated and contribute to the inflammatory response through releasing a wide variety of mediators including cytokines (IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-16, IL-18, tumor necrosis factor-α [TNF-α] and transforming growth factor-α and β [TGF-α and TGF-β]), chemokines (MIP-1, MCP-2, RANTES and eotaxin), and lipid mediators (PGE1, PGE2, thromboxane B2, PAF, LTC4) (Fig. 44-2).26,27 Eosinophils secrete granule proteins, which are important in the eosinophil’s primordial role as the primary defender against parasites, as well as in the pathogenesis of asthma.26,28 Eosinophils contain both primary and secondary granules. The primary granules contain Charcot–Leyden crystal protein, while the secondary granules contain the four principal cationic proteins: major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), and eosinophil peroxidase (EPO).26 These cationic proteins play various roles in the pathogenesis of asthma including the induction of mast cell and basophil degranulation (ECP and MBP), increasing airway mucus production (ECP), and formation of reactive oxygen species (EPO) (Fig. 44-2).26
Figure 44-2 Eosinophil products in asthma. EPO, eosinophil peroxidase; EDN, eosinophil-derived neurotoxin; ECP, eosinophil cationic protein; MBP, major basic protein; PGE2, prostaglandin E2; TBXA2, thromboxane A2; PAF, platelet activating factor; LTC4, leukotriene C4; RANTES, regulated upon activation normal T cell expressed and secreted; MCP-2, monocyte chemotactic protein 2; MIP-1, macrophage inhibitory protein 1; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor beta.
Over the past 30 years, the role of the eosinophil in asthma has undergone considerable re-evaluation. Since the discovery of the eosinophil by Ehrlich in 1879 and the later discovery that Ehrlich’s cells were present in the sputum of asthmatic patients, the eosinophil has been viewed as the primary effector cell in asthma.29 Studies have noted that peripheral blood eosinophilia is a characteristic of asthma and often in relationship to disease severity, and that eosinophilic infiltrates were found in the airways of asthma patients at autopsy, regardless of whether asthma was the primary cause of death.30,31 Later studies detected increased eosinophils and eosinophil products in bronchoalveolar lavage (BAL) fluid after antigen challenge.32 The view that the eosinophil was the principal effector of asthma was largely unchallenged until the new millennium brought new therapies, including monoclonal antibodies to IL-5, into evaluation.
The importance of IL-5 to the differentiation and survival of the eosinophil has been previously noted. Initial studies with monoclonal antibodies to IL-5 in asthma showed a decrease in sputum and peripheral blood eosinophilia, as expected, but failed to demonstrate significant benefit in a wide variety of clinical outcome measures, that is, symptoms or improved airflow obstruction.33,34 Although this lack of effect on parameters of clinical asthma was a surprise, it led to an increasing interest in the heterogeneity of asthma with the realization that eosinophils may play greater or lesser roles in different patients.
Later studies with anti-IL-5 were conducted in patients who had persistent eosinophilia despite treatment with inhaled corticosteroids (ICS), and in these patients were noted clinical outcomes, the prevention of exacerbations.35,36 Eosinophils play an important role in a certain subset of asthma, but the contribution of their role appears to be defined by phenotypes and is not necessarily generalizable to the entire asthmatic population. Finally, eosinophils are likely a prime contributor to airway remodeling seen in chronic asthma, and will be discussed in this capacity later in the chapter.
Neutrophils
Neutrophils are granulocytes derived from CD34+ hematopoietic stem cells and are normally present in the bloodstream, as well as in various tissues, including the lung. They contain primary (azurophilic) and secondary (specific) granules, which contain a variety of antimicrobial enzymes, neutral proteases, and acid hydrolases.37 Neutrophils are attracted to the airway by various cytokines and chemokines including IL-8, IL-17, and granulocyte colony stimulating factor (G-CSF).38,39 Airway neutrophilia can be seen in many respiratory conditions, viral respiratory infections, COPD, and asthma.10,39
The role of neutrophils in response to viral respiratory infections was introduced previously. In response to inoculation with a respiratory virus, such as HRV, dendritic cells, and other mononuclear cells produce proinflammatory cytokines and chemokines, which recruit neutrophils to the airway.10 Neutrophils contribute to the inflammatory milieu by secreting cytokines such as TNF-α, IL-1, IL-8, and IL-18, to attract other inflammatory cells, upregulate cytokine production, and produce airway inflammation and enhance bronchial hyperresponsiveness.40 Neutrophil products, such as elastase, can have more direct effects on the airway and cause mucus production.10 In addition, because neutrophils are found in severe asthma, they are proposed to play a more prominent role in this phenotype.41 Prominent neutrophilic inflammation has been noted in the sputum of patients with severe asthma exacerbations, in BAL fluid from patients with noninfectious status asthma, and in autopsy specimens from the airway in patients with acute, fatal asthma.42–44 Other studies have demonstrated subgroups of patients with chronic asthma in whom the primary inflammatory cell type is neutrophils rather than eosinophils.45,46 These patients are often more difficult to treat and less responsive to treatment with corticosteroids.
Lymphocytes
Unlike the previously discussed cell types, T cells are lymphocytes and derived from the common lymphoid progenitor. Numerous subsets of T cells have been identified and are important contributors to asthma, including CD4+ helper T cells and their subsets (TH1, TH2, TH9, and TH17), CD8+ cytotoxic T cells, and regulatory T cells (TREG).
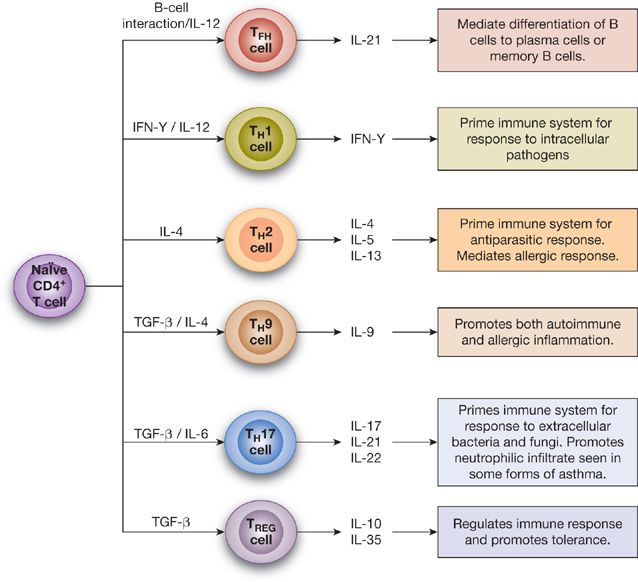
CD4+ helper T cells recognize antigens presented to them by antigen presenting cells (APCs) and, in turn, secrete cytokines to influence the inflammatory response. In the airway, the dendritic cell is the most important APC and its role will be described later. A complex series of events involving cytokines and various transcription factors determines whether CD4+ T cells will differentiate into TH1 cells, TH2 cells, TH9 cells, or TH17 cells (Fig. 44-3).47
Figure 44-3 CD4+ T Lymphocyte subsets. IFN-γ, interferon gamma; TGF-β, transforming growth factor beta; TFH cell, T follicular helper cell; TREG cell, regulatory T cell.
TH2 cells are recognized as the primary drivers of inflammation in asthma and allergic disease. When TH2 cells encounter antigen presented by dendritic cells, they produce IL-4, IL-5, and IL-13, all of which play critical roles in the pathogenesis of asthma and are part of the clinical disease as demonstrated by detection of increased levels of these cytokines in the BAL fluid of patients with asthma.48,49 IL-4 increases IgE production by plasma cells. As mentioned previously, IL-5 is critical in the terminal differentiation and homing of eosinophils to the airway. IL-13 also increases IgE production and plays a prominent role in airway hyperresponsiveness and tissue remodeling.50
The role of TH1 cells in asthma is not as well defined as TH2 cells. Although it has been presumed that TH1 cells counteract the asthma-inducing effects of TH2 cells, this likely is an over-simplification. TH1 products have been shown to be increased during asthma exacerbation.51 Some studies have also suggested that TH1 cells may play a more prominent role in chronic severe asthma as evidenced by increased levels of the primary TH1 cytokine, interferon-γ (IFN-γ), in the BAL fluid of patients with severe asthma.52
TH9 cells are a relatively newly defined cell population whose primary cytokine is generation of IL-9. TH9 cells function similarly to TH2 cells in that they increase allergic inflammation. Mast cells are the primary IL-9 receptor-bearing cell, and TH9 cells are important contributors to the previously described increased mast cell activation seen in asthma.20 Because mast cells also produce VEGF and fibroblast growth factor-2, TH9 cells likely contribute to airway remodeling seen in chronic asthma.20
The role of TH17 cells in asthma is an area of intense research. With the identification of subphenotypes of asthma patients with primarily neutrophilic inflammation, the role of IL-17 (the primary cytokine produced by TH17 cells) in asthma has become of considerable interest. Although IL-17 is found to be increasingly expressed in patients with severe asthma,53 it is, however, also found in high concentrations in patients with mild asthma (FEV1 >70% predicted) and these values correlate negatively with the PC20.54 Thus, while TH17 cells most certainly play a role in patients with primarily neutrophilic asthma, they are also likely important in milder forms of disease as well.
The primary function of CD8+ cytotoxic T cells is the destruction of human cells that are infected with viruses or other intracellular pathogens. CD8+ T cells also likely play a role in asthma, though the extent of their contribution has yet to be fully elucidated.50 IL-4 and IL-5 producing CD8+ T cells are present in the airways of asthmatic patients.55 IL-5 production by CD8+ T cells is increased in the presence of a viral respiratory infection, and the overall cytokine production by CD8+ T cells correlates with asthma severity.56,57 Whether CD8+ T cells function as direct contributors or bystanders in the worsening of asthma has yet to be determined.50
TREG cells also appear to play a critical role in asthma development. TREG cells serve to limit inflammatory responses and promote immune tolerance through the production of IL-10 and TGF-β.50,58 In patients with asthma and other allergic disorders, TREG cells appear to be less effective in limiting TH2 inflammation.59,60 However, after allergen immunotherapy, for example, TREG cells increase in the nasal mucosa and may act to promote allergen tolerance.61 Interestingly, farm exposure early in life is associated with a decreased incidence of allergic disease and asthma, a fact that may be related to increased numbers and function of TREG cells in infants living in this environment.62
Natural killer cells (NK cells) are members of the innate immune system and serve as a first line of defense against infections. Their role in the pathogenesis of asthma has yet to be fully elucidated. They obviously appear in response to viral respiratory infections, and NK cells increase during asthma exacerbations.63 NK cells are capable of producing numerous cytokines including IFN-γ, IL-4, IL-5, and IL-13.64 NK cells from patients with atopic asthma are skewed toward the production of IL-4 as opposed to IFN-γ upon activation.64 IFN-γ production by NK cells is also inhibited by prostaglandin D2, a TH2-promoting lipid mediator produced by mast cells.65 NK cells may also play a role in “dendritic cell editing” by killing immature dendritic cells, which might influence a certain type of TH response.66
Macrophages and Dendritic Cells
Macrophages and dendritic cells are descendants from the CD34+ hematopoietic stem cell and arise from a common committed precursor cell.67 Macrophages arise from circulating monocytes and function, primarily, to clear debris and microbes from the airway. They may also function as antigen presenting cells, although this role is likely less important than that of the dendritic cell.68 Alveolar macrophages may further differentiate into M1 or M2 subsets based on exposure to various cytokines and toll like receptor (TLR) agonists.67 M1 macrophages are “classic” macrophages, and clear microbes from the airway. They also produce cytokines, such as IL-12, IL-6, and TNF-α, as well as high levels of nitric oxide (NO).67 M1 macrophages have traditionally been described as suppressing allergic inflammation, primarily through their secretion of TH1 cytokines such as IL-12; this is not fully resolved.67
Differentiation into M2 macrophages is influenced by an environment rich in TH2 cytokines such as IL-4 and IL-13, thus implicating their role in asthma. When compared with M1 macrophages, M2 macrophages are poor at clearing intracellular pathogens.67 They release cytokines such as IL-13 and thus are likely contribute to the airway hyperresponsiveness.67
Dendritic cells are the lung’s primary presenter of antigen to T cells. Their role as the primary APC places the dendritic cell at a critical junction in determining what type of T cell response will be directed toward the antigen (i.e., TH1, TH2). Dendritic cells in humans exist in two broad categories: the myeloid dendritic cell (mDC) and the plasmacytoid dendritic cell (pDC). While both types of dendritic cells are present in the human lung, their anatomic localization within the lung is poorly understood. 5 Upon encountering antigen in the airway, dendritic cells migrate to local lymph nodes where they present antigen to T lymphocytes. Both pDC and mDC levels increase in the airway (and coincidently decrease in the blood) after exposure to inhaled allergen.69–71 Because dendritic cells lie close to the epithelial barrier, they receive numerous signals from epithelial cells, which can influence their effect on T cells. TSLP is produced by epithelial cells and promotes dendritic cells to direct TH2 differentiation and recruit TH2 cells to the airway.5 Other epithelial cell–derived factors, such as GM-CSF, TNF-α, CCL-20, IL-1β, and TNF-related apoptosis-inducing ligand (TRAIL), have similar TH2 promoting effects.5 pDC represent the lung’s primary source of IFN-α, a potent antiviral cytokine, and they are recruited to the lung during times of viral infections.72 As viral infections commonly precede asthma exacerbations and may predispose infants to develop asthma, this role of the pDC cannot be understated.73 The importance of pDC to the development of asthma was illustrated by a recent study which showed that decreased pDC levels in childhood was directly correlated to increased number and severity of viral respiratory infections, increased episodes of wheeze, and increased asthma diagnosis.74 The function of mDC is not as well known in human asthma, but the role of both dendritic cell types in contributing to the development and propagation of the disease will continue to be an important area of asthma research.
 RESIDENT CELLS OF THE AIRWAY
RESIDENT CELLS OF THE AIRWAY
The roles of airway smooth muscle cells and airway epithelial cells in the biology of asthma are considered below.
Airway Smooth Muscle
Given that bronchospasm and bronchial constriction are critical components of asthma, it is intuitive that the cell type responsible for this component, the airway smooth muscle cell, would be a key factor in asthma pathogenesis and pathophysiology. While its importance seems obvious, the details of why airway smooth muscle function is so different in the asthmatic airway compared with the normal airway have been elusive. It has been well documented that the smooth muscle layer surrounding the airway is thicker in asthma compared to nonasthmatic controls. These difference are due to both smooth muscle hypertrophy and hyperplasia.75 Increased inflammatory cells, including mast cells, can be found within the smooth muscle bundles of asthmatic airways, and the interplay between these cells, the airway epithelium, and the smooth muscle layer, is an important determinant of the asthmatic response.17 Numerous cytokines, chemokines, and growth factors are involved in this interaction including those produced by the airway smooth muscle cell itself and those produced by other cell types with which the airway smooth muscle cell communicates. Airway smooth muscle cells in asthma are more prolific producers of these cytokines, and this function likely plays a role in their ability to proliferate more rapidly than those of nonasthmatic subjects.76
The treatment of asthma has long focused on preventing or reversing contraction of bronchial smooth muscles. β2-agonists, both short and long acting, have long been key to the treatment of asthma, and these medications act directly on the airway smooth muscle. More recently, debulking of airway smooth muscle, with the use of bronchial thermoplasty, has shown improvement in asthma control, which, in theory, further supports the role that airway smooth muscle plays in asthma pathophysiology.77,78
Airway Epithelial Cells
The epithelial lining of the airway is an area of intense research, and its importance, beyond being a simple anatomic barrier, is being increasingly recognized and appreciated. The airway epithelium represents a vast surface area (100 m2) that is in contact with some 10,000 L of inhaled air daily.79 As the area of initial contact between the lung and the external airborne environment, functions of the airway epithelium are likely key to determine the body’s response to airborne substances.
The airway epithelium is composed of three major cell types: the ciliated columnar epithelial cell, the mucus-secreting goblet cell, and the surfactant secreting Clara cell.79 Both overproduction of mucus by goblet cells and underproduction of important anti-inflammatory peptides by Clara cells have been noted in patients with asthma.80,81 The tight junctions between epithelial cells have also been noted to be defective in patients with asthma, which can serve to decrease the ability of the airway epithelium to act as a protective barrier.79 The airway epithelium in asthma is also less capable of defending itself against reactive oxygen species, a defect that leads to further damage to this airway barrier.82 Epithelial cells in asthma produce lower amounts of Type I interferon in response to respiratory viruses, which can increase the severity to respiratory infections and promote asthma exacerbations.83 TSLP promotes TH2-driven inflammation and is overproduced by epithelial cells in asthmatic patients, thus providing a crucial link between the airway epithelium and the TH2 type inflammation seen in asthma.84,85 Finally, epithelial cells are capable of secreting endothelins, which are peptides with significant bronchoconstrictive activity.86
MOLECULAR MEDIATORS IN ASTHMA
The above-mentioned cells are able to initiate, perpetuate, coordinate, and regulate the inflammatory process with the synthesis and secretion of several different classes of molecular mediators. These mediators have a variety of functions, as discussed below.
 CYTOKINES
CYTOKINES
Cytokines are small–molecular-weight glycosylated signaling molecules that are secreted by a number of different cell types with autocrine, paracrine, or endocrine directive activities.87 Cytokine is a broad term and includes many subcategories including interleukins, interferons, and growth factors.87 Cytokine secretion is usually a brief, self-limited event. It may, however, require new mRNA and protein synthesis, which takes place over a matter of hours rather than seconds or minutes.88 A variety of cytokines have been implicated in the regulation of airway inflammation and thus in the pathogenesis of asthma (Table 44-1).89 The support for cytokine involvement in inflammation was first obtained by the detection of these mediators in the airways of patients with asthma, particularly in bronchoalveolar lavage fluid after allergen challenge and in situ hybridization of retrieved cells or biopsy materials.90,91