Treatment of Non–Small-Cell Lung Cancer: Chemotherapy
Historically, lung cancer is associated with high mortality rates and little effective therapy. Because this disease predominately affects those of advanced age with significant comorbidities, treatment can be difficult to deliver safely with manageable adverse effects. All of these factors can lead to a sense of futility among clinicians and patients when discussing lung cancer therapy. However, in the last several years novel therapies have emerged to make lung cancer therapy better tolerated and more effective, even among those with significant comorbidities. The introduction of better-tolerated cytotoxic chemotherapy and targeted agents has made lung cancer therapy tenable for many more patients. In addition, the improved response rates seen by matching targeted drugs to specific genetic alterations driving tumor growth have led to improved quality of life and survival among patients with these specific tumors. Thus, although the morbidity and mortality of lung cancer remain high, novel approaches to therapy and improved supportive care have begun to make a significant impact in the burden of this disease.
Chemotherapy, whether an oral targeted drug or an intravenous cytotoxic agent, is used for three main reasons in the treatment of non–small-cell lung cancer (NSCLC): (1) As adjuvant therapy in early-stage disease following potentially curable surgical resection to prevent disease recurrence, (2) as concurrent therapy with radiation in locally advanced disease to radiosensitize the tumor and prevent metastatic disease recurrence, and (3) as palliative therapy in the setting of advanced disease to ease symptoms and prolong survival. This chapter will focus on the role of systemic chemotherapy in the treatment of NSCLC in each of these settings.
EARLY-STAGE NON–SMALL-CELL LUNG CANCER
Surgery remains the standard of care for patients with early-stage disease who do not have medical contraindications. In this setting, surgery provides definitive treatment and allows for more accurate pathologic staging. Staging is central to the therapeutic approach to NSCLC. This entails determination of the extent of invasion of the mediastinal lymph nodes. Mediastinoscopy or fine-needle aspiration (FNA) of lymph nodes by endobronchial ultrasound (EBUS) can be used to sample mediastinal lymph nodes before a surgical resection. As for all surgical interventions for thoracic malignancy, complete nodal sampling or lymph node dissection is an integral part of the procedure. Reliance on noninvasive imaging alone may be inadequate for accurate assessment of the mediastinum (Fig. 114-1).
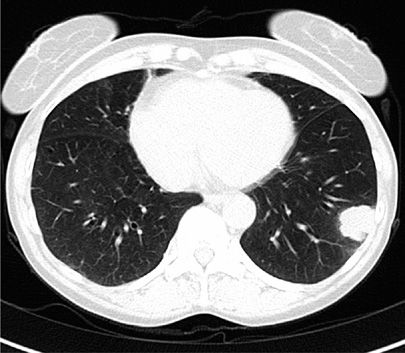
Figure 114-1 Computerized tomography (CT) scan of a 59-year-old female with ongoing smoking and chronic cough. The image shows a large nodule in the left lower lobe extending into the pleura. The patient underwent a mediastinal lymph node dissection and left lower lobectomy. She was found to have several hilar lymph nodes involved with tumor at the time of resection, (stage IIA). She received four cycles of cisplatin-based adjuvant chemotherapy following surgical resection.
 ADJUVANT CHEMOTHERAPY
ADJUVANT CHEMOTHERAPY
It is well recognized that despite complete resection, most patients with locally advanced NSCLC will develop disseminated disease. The risk of developing disseminated disease can be predicted, with some accuracy, on the basis of the stage of the disease determined at the time of the initial resection. However, the value of the staging information depends on the completeness of the staging procedures carried out at the time of resection. Even with stage I disease, as many as 20% of patients die of disseminated disease within 5 years. With stage II disease, less than 50% of patients are alive at 5 years; with stage IIIA (N2) disease, at best, 30% of patients are alive at 5 years. These numbers make clear the need for additional therapy to improve on the overall survival (OS) achieved by surgery.
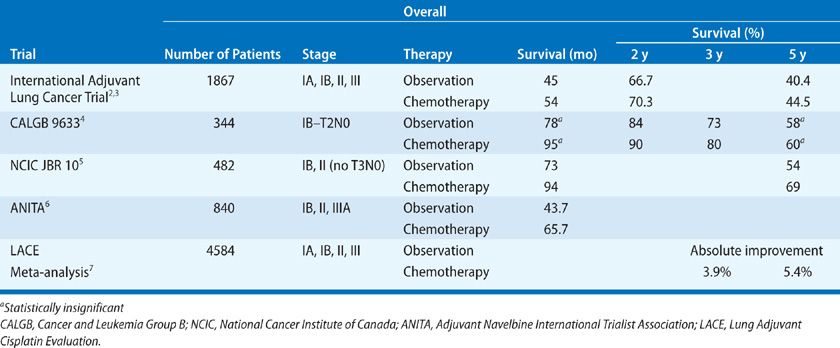
To this end, there has been an emergence of a body of data to better define the role of chemotherapy in the adjuvant setting. The rationale behind this approach is to treat patients who are deemed to be at high risk for recurrence and dissemination of disease in the hope of eliminating micrometastatic disease. Early trials to study adjuvant chemotherapy were negative, possibly either due to the use of less effective chemotherapy regimens, the increased morbidity of treatment with fewer supportive care options, or the lack of statistical power. However, interest in studying adjuvant chemotherapy reemerged with presentation of a meta-analysis in 1995 in which 52 randomized trials were reviewed.1 The authors concluded that the trials that compared cisplatin-based adjuvant chemotherapy to no further treatment favored the use of chemotherapy with an absolute survival benefit at 5 years of 5%. Since then, more homogeneous trials randomizing patients to surgery versus surgery followed by platinum-based chemotherapy have been conducted. These studies are outlined in Table 114-1.
International Adjuvant Lung Cancer Trial
The International Adjuvant Lung Cancer Trial (IALT) randomized 1867 patients with stage I, II, or III NSCLC to observation or chemotherapy after surgical resection.2 The two groups were evenly matched with regard to age, sex, stage, performance status (PS), type of surgery, and histologic subtype. Due to the lack of consensus regarding the use of adjuvant radiotherapy, the choice of specific regimen was made by each participating institution at the time of protocol development. The chemotherapy regimens used consisted of cisplatin 80, 100, or 120 mg/m2 offered on one of four different schedules with vindesine, vinblastine, vinorelbine, or etoposide. Patients were treated with three or four cycles. Of the various choices, the combination of cisplatin and etoposide was used to treat nearly 50% of the patients. When offered, radiation was given after chemotherapy in the group randomized to postoperative treatment. Radiation was planned for about 30% of the patients in the trial, with two-thirds of these having N2 disease. It was actually delivered to slightly more patients in the control group compared with the chemotherapy group (28% vs. 23%). Overall, the results favored the use of adjuvant chemotherapy, with a hazard ratio (HR) for death of 0.86 (0.76–0.98). At 5 years, the group that received chemotherapy had a statistically significant improvement in survival of 44.5% versus 40.4%. The toxicities associated with chemotherapy included the expected risks of neutropenia and nausea/vomiting. However, seven patients, (0.8%) died due to chemotherapy-related acute toxicities; this was predominately seen in those who received cisplatin at 120 mg/m2. Long-term follow-up, (median 7.5 years) of this study was reported in 2009.3 Although the benefit of cisplatin-based adjuvant chemotherapy on disease recurrence did not decrease with long-term follow-up, there was an increase in nonlung cancer–related death seen after 5 years in the treatment group. This positive trial demonstrated an absolute benefit at 5 years that was consistent with the previous meta-analysis and provided support for the use of cisplatin-based treatment in the adjuvant setting.
Retrospectively, tissue samples collected from patients participating in the IALT have been analyzed by immunohistochemistry to assess which biomarkers may predict a response to cisplatin therapy. Specifically, investigators have assayed for the enzyme excision repair cross-complementation group 1 (ERCC1).8 Cisplatin acts by directly binding to DNA and forming platinum-DNA adducts, which prevents DNA replication. It is known that the presence of high levels of ERCC1 is associated with cisplatin resistance due to DNA repair of cisplatin adducts. To study this in the context of the IALT results, 761 tumor samples from that trial were assayed for ERCC1 expression by immunohistochemistry; half of these patients received chemotherapy and the remainder was in the control group. Of the tumors analyzed, 44% were ERCC1 positive. Expression was more common in patients over the age of 55 and those with squamous cell histology. Notably, there was a benefit from adjuvant chemotherapy in patients with ERCC1-negative tumors, with a statistically significant improvement in OS and disease-free survival due to chemotherapy (HR 0.65). In contrast, patients with ERCC1-positive tumors did not achieve a survival benefit from adjuvant chemotherapy compared with the control group. Among patients in the control group, those with ERCC1-positive tumors had an increased survival compared with patients with ERCC1-negative tumors. Although these results are intriguing, ERCC1 levels are difficult to quantify and it is currently not routinely tested in the clinical setting. Its use as a predictive marker for cisplatin-based adjuvant therapy needs to be validated in a prospective clinical trial.
CALGB 9633
The Cancer and Leukemia Group B (CALGB) conducted a trial to test the benefit of carboplatin-based adjuvant chemotherapy exclusively in stage IB (T2N0) patients.4 Other studies have included these patients along with those with more advanced disease, and in subset analyses the true benefit of chemotherapy after surgery in this population has been questioned. Originally, this study was intended to enroll 500 patients. However, due to poor accrual, this number was modified and in the final analysis, 344 patients were treated. Patients randomized to the chemotherapy arm received carboplatin dosed to a target area under the curve (AUC) of 6 mg/mL/min and paclitaxel 200 mg/m2 every 3 weeks for four cycles. This study has been provocative in that it was initially reported in 2004 to be a positive trial with a statistically significant survival advantage, after a median follow-up of 34 months. This prompted the study to be terminated early, and the National Comprehensive Cancer Network (NCCN) practice guidelines adopted the use of adjuvant chemotherapy in stage IB patients. In 2008, when the results were updated after longer follow-up, (mean 74 months), the difference in 5-year survival between the chemotherapy and observation arms (59% vs. 57%, HR 0.83) was no longer statistically significant. However, the 3-year survival difference remained statistically significant, and there was a trend favoring a benefit in OS in the patients who received chemotherapy. Despite this, the routine practice of treating patients with stage IB disease is no longer recommended. In a subset analysis, there was a statistically significant benefit overall, (HR 0.69, p = 0.43), in patients with tumors over 4 cm in size who received chemotherapy. This benefit was not shared in those with tumors less than 4 cm in size. Therefore, in practice, many oncologists choose to treat patients with large-stage IB tumors, based on this subset analysis. The reason why the results are negative is not known and may be due to the population of patients being treated, the choice of a carboplatin-based regimen rather than cisplatin, or the abridged number of patients who were treated.
NCIC JBR 10
In this intergroup study, 482 patients with stage IB or II (excluding T3N0) NSCLC were randomized to either surgery or surgery followed by four cycles of chemotherapy.5 Again, the treatment studied included cisplatin (50 mg/m2 days 1 and 8) and vinorelbine (25 mg/m2 weekly); one cycle was 4 weeks. This also was a positive study, with the 5-year survival favoring the chemotherapy group (69% vs. 54%), as well as a statistically significant improvement in disease-free and OS. Toxicity was associated with this regimen with two treatment-related deaths and over 70% of patients experiencing either grade 3 or 4 neutropenia. Other toxicities included fatigue, anorexia, and vomiting.
ANITA
The most recent study of interest is the Adjuvant Navelbine International Trialist Association (ANITA) study, in which 799 patients with stage IB, II, or IIIA NSCLC were randomized to four cycles of chemotherapy or observation after surgery.6 Similar to the other trials, the chemotherapy regimen consisted of cisplatin (100 mg/m2, day 1) and vinorelbine (30 mg/m2, weekly) for a 4-week cycle. The choice of radiotherapy was left to the discretion of the treating physicians, and in the final analysis 24% of patients in the chemotherapy arm and 33% of patients in the observation arm received radiation. Patients who were randomized to chemotherapy had a significant improvement in median survival to 66 months, compared with 44 months in the control group. At 5 years, the absolute benefit in survival was 8.6%, and in a subset analysis this benefit seemed to be mainly noted in stage II and IIIA patients.
Lung Adjuvant Cisplatin Evaluation Meta-Analysis
In 2008, the Lung Adjuvant Cisplatin Evaluation (LACE) Collaborative Group published results of a pooled analysis of large trials (>300 subjects) investigating the use of cisplatin-based adjuvant chemotherapy versus no further therapy following complete resection of NSCLC.7 The individual data from over 4500 subjects was analyzed with a median of 5.2 years follow-up. The investigators found an absolute benefit of 5.4% improvement in OS at 5 years in the treatment group. There was no interaction seen in regards to gender, age, histology, or type of surgery. However, the degree of benefit varied with stage; the HR for stage IB was 0.93 (95% CI: 0.78–1.10), for stage II HR = 0.83 (95% CI: 0.73–0.95), and for stage III, HR = 0.83 (95% CI: 0.72–0.94). In this analysis, a significant benefit from adjuvant chemotherapy was seen in those with better PS. In patients with Eastern Cooperative Oncology Group (ECOG) PS of 2 – defined as “ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours,” – chemotherapy may be detrimental and is not recommended. This analysis confirmed the benefit of cisplatin-based adjuvant chemotherapy following complete resection in fit patients with stage II and III NSCLC. Currently the NCCN and American Society of Clinical Oncology (ASCO) guidelines recommend cisplatin-based adjuvant chemotherapy for stage II and IIIA NSCLC.
Future Directions
Adjuvant cisplatin doublet chemotherapy is currently the standard of care for stage II and IIIA patients. The role of adjuvant treatment of stage IB patients has yet to be defined more clearly, but based on subset analyses, it is possible that patients with tumors greater than 4 cm will benefit from chemotherapy. Given the now-negative results of the CALGB 9633 study, carboplatin cannot be recommended as a standard choice for adjuvant treatment. Hopefully future studies will provide further data to guide the therapy of stage IB patients. Also, although molecularly targeted agents have been studied in the advanced disease stage (as detailed in the following sections), the utility of these agents in the early-stage setting is unknown. To begin to answer this question, the current large intergroup effort that is underway will randomize patients with stage IB (tumor >4 cm), II, and IIIA NSCLC to a cisplatin-based doublet either with or without the antiangiogenesis agent bevacizumab. Adjuvant trials are also underway to assess the role of erlotinib in patients with a sensitizing mutation in EGFR.
LOCALLY ADVANCED NSCLC
The term locally advanced includes several different presentations of primary lung cancer, but all have in common the absence of disease outside of the chest. Included in this category are those with stage IIIA or IIIB disease. These tumors involve mediastinal lymph nodes, either ipsilateral (N2) or contralateral (N3), and/or large primary tumors that directly invade important thoracic structures. For example T3 tumors involve the chest wall, the main bronchus less than 2 cm from the carina, or are greater than 7 cm in size. T4 tumors invade the mediastinum and affect structures that are not usually considered resectable (e.g., aorta, esophagus, and vertebral bodies). Mediastinal lymph node invasion should be determined before surgical resection by way of mediastinoscopy or EBUS with FNA, which also allows contralateral mediastinal lymph nodes to be sampled.
About 40,000 cases of stage IIIA and IIIB disease occur per year in the United States. The best treatment approach to locally advanced disease has not yet been determined. A wide array of combined modality approaches have been used in stage IIIA patients (particularly those with N2 nodes). These include varying combinations of chemotherapy, radiation, and surgery. Many studies in which patients with locally advanced disease were treated with chemotherapy, radiotherapy, or a combination of the two have relied on noninvasive determination of the extent of the disease. Thus, on the basis of enlarged ipsilateral mediastinal lymph nodes seen on a computed tomographic (CT) scan, patients were assumed to have N2 disease and were treated without histologic documentation of mediastinal lymph node impairment. Such studies are seriously flawed. For meaningful interpretation, accurate histologic staging has to be included as an entry criterion for any study of locally advanced disease.
 LOCALLY ADVANCED INOPERABLE OR UNRESECTABLE STAGE III NSCLC
LOCALLY ADVANCED INOPERABLE OR UNRESECTABLE STAGE III NSCLC
Below are considered treatment approaches based on sequential or concurrent chemotherapy and radiation therapy in the management of locally advanced, inoperable or unresectable Stage III NSCLC.
Chemotherapy Followed by Radiation Therapy
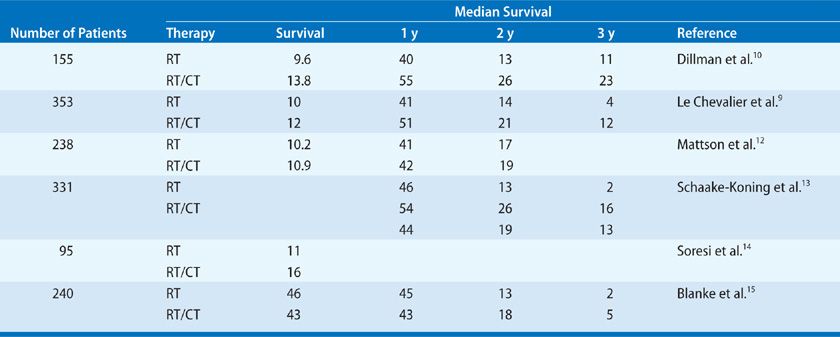
Several prospective, randomized studies have compared radiation therapy alone and radiation therapy in sequence with chemotherapy in the setting of unresectable stage III disease. Le Chevalier et al.9 randomized 353 patients to radiation alone (6500 cGy) or to cisplatin-based chemotherapy followed by radiation. One-, 2-, and 3-year survival rates all favored the combined therapy arm (51%, 21%, and 12% vs. 41%, 14%, and 4%, respectively). However, using repeat biopsies, the study found only a 17% incidence of local control in the radiation arm and a 15% incidence of local control in the chemotherapy and radiation arm.
In a trial conducted by CALGB, 155 patients were randomized to either radiation alone (6000 cGy) or a cisplatin-based regimen followed by radiation.10 The median survival favored the combination therapy arm (13.8 vs. 9.7 months). The results at 1 and 2 years were so striking for the combined therapy group that the study was terminated early—a decision that subsequently prompted considerable criticism. The 3- and 5-year survival rates also favored the combination therapy (25% and 19%) over radiation therapy alone (11% and 7%). Unfortunately, the study was limited to patients with a high PS and less than 5% weight loss in the 6 months before enrollment in the trial. Limiting a study to the most favorable patients begs the question of the applicability of the results to the general group of patients with locally advanced lung cancer, many of whom have a decrease in their PS and have lost considerable weight.
A study seeking to confirm the CALGB report was initiated by the Radiation Therapy Oncology Group (RTOG), which randomized patients to the same two arms, in addition to a third arm using hyperfractionation radiation (69.6 cGy twice daily fractions) as the only treatment modality.11 This study demonstrated that chemotherapy given prior to radiation was indeed superior to radiation alone in the patients with good PS (i.e., loss of weight of less than 5% in the previous 3 months). Analysis at 1 year showed the median survival to be statistically longer for those in the combined chemotherapy and radiation arm. At 3 years follow-up, however, no difference in survival (14%) was observed between the chemotherapy and radiation arm and the hyperfractionated arm. Both of these treatment regimens were better than standard radiation alone (Table 114-2).
TABLE 114-2 Randomized Trials in Stage III Disease: Radiation Alone Versus Radiation and Chemotherapy
Concurrent Chemotherapy and Radiation Therapy
The rationale for concurrent therapy, that is, chemotherapy given during a course of radiation therapy, is based on the concept that some drugs or drug combinations (notably cisplatin) may increase the radiosensitivity of tumor cells. The trade-off, however, is an increase in toxicity and a regimen that is not well tolerated by all potentially eligible patients. A meta-analysis of randomized trials directly comparing platinum-based chemotherapy given concurrently with thoracic radiation to chemotherapy given sequentially with thoracic radiation was performed in 2010.16 Data from six trials (1205 individual patients) were analyzed. There was a significant benefit to concurrent therapy with an absolute OS benefit of 5.7% at 3 years (HR = 0.84, 95% CI: 0.74–0.95, p = 0.004). The primary effect was seen in control of locoregional disease, with no difference in risk of distant recurrence over sequential therapy. The risk of severe esophagitis was increased with concurrent therapy, although pulmonary toxicity was not different. Concurrent platinum chemotherapy and radiation therapy is the treatment of choice for patients with locally advanced, inoperable disease, provided their PS and comorbidities do not limit their ability to withstand the toxicities associated with this approach. Based on small phase II trials conducted by the Southwest Oncology Group (SWOG), a favored regimen for concurrent therapy utilizes cisplatin (day 1, 8, 29, 36) and etoposide (days 1–5, 29–33) with conventional RT.17–19 For those patients unable to receive cisplatin, due to renal insufficiency or hearing loss, weekly carboplatin and paclitaxel has demonstrated activity in this setting. The OS seen with carboplatin and paclitaxel treatment is less than the control arms of the cisplatin/etoposide studies, although these two chemotherapy regimens have not been directly compared in a randomized trial. For those unable to tolerate a concurrent approach, sequential chemotherapy followed by radiation is a reasonable alternative.
Induction or Consolidation Chemotherapy in Addition to Concurrent Chemoradiation
Although the addition of concurrent platinum-based chemotherapy to thoracic radiation for unresectable stage III NSCLC has been proven to improve local control and OS, disease recurrence at distant sites remains high. With concurrent therapy, only two cycles of chemotherapy are given during the course of radiation. This is fewer than the four cycles of cisplatin-based chemotherapy that has proven benefit to lessen disease recurrence in the adjuvant setting. This has led to an interest in increasing the amount of chemotherapy given for patients receiving concurrent therapy in locally advanced disease by adding several additional cycles of chemotherapy either prior to (induction) or after (consolidation) the concurrent chemoradiation.
Multiple randomized clinical trials have studied each of these approaches. In a nonrandomized phase II study, concurrent chemoradiation was followed by two cycles of consolidation cisplatin and etoposide, resulting in a median survival of 15 months and a 5-year survival of 15%.18 A subsequent trial used the same combined treatment regimen, but then switched to docetaxel for consolidation.19 This yielded a median survival of 26 months and a 3-year survival of 37%. However, a randomized study performed by the Hoosier Oncology Group in which patients received cisplatin, etoposide, and radiation, either with or without consolidation docetaxel, failed to show a benefit from consolidation treatment.20 This is the first and only randomized study of consolidation therapy after concurrent therapy and radiation. Another favored regimen utilizes low-dose carboplatin and paclitaxel with radiation, followed by consolidation therapy with the same agents. Noncomparative phase II studies of this regimen have demonstrated a median survival of 16 months.21 CALGB published findings of a phase III randomized study of two cycles of induction chemotherapy with full-dose carboplatin and paclitaxel followed by concurrent weekly dosing with radiation versus concurrent therapy alone.22 Overall survival was not significantly improved with induction therapy (14 vs. 12 months, p = 0.3). Multiple studies have attempted to integrate targeted agents into the treatment of patients with unresectable, locally advanced disease, as consolidation following chemotherapy and radiation, with disappointing outcomes to date.
New Approaches to Concurrent Chemoradiation
Novel approaches to the treatment of locally advanced, nonresectable NSCLC include addition of pemetrexed and/or the monoclonal antibody, cetuximab as radiosensitizers and increasing the dose of radiation to 74 Gy. In 2011, the results of CALGB Trial 30407, a phase II study of pemetrexed and carboplatin administered with concurrent radiation ± cetuximab, were reported.23 The combination of pemetrexed, carboplatin, and concurrent radiation with an additional four cycles of pemetrexed alone as consolidation met the primary end point of an 18-month survival rate of 58% and was recommended for further study; the addition of cetuximab did not meet statistical significance. The results of PROCLAIM, a phase III study of pemetrexed, cisplatin, and concurrent radiation with pemetrexed consolidation versus cisplatin and etoposide with concurrent radiation and consolidation is awaited. This study will determine if pemetrexed improves outcomes in stage IIIB in those patients with predominately nonsquamous histology. Finally, results of the RTOG 0617 study randomizing patients to standard dose (60 Gy) versus high dose (74 Gy) of chemoradiation ± cetuximab are awaited. The initial planned interim analysis for OS was recently reported, with discontinuation of the high-dose radiation arm due to inferior survival.23 The results of the addition of cetuximab are pending.
 LOCALLY ADVANCED RESECTABLE NSCLC
LOCALLY ADVANCED RESECTABLE NSCLC
The roles of sequential chemotherapy followed by surgery and concurrent chemoradiation followed by surgery in treating locally advanced, resectable NSCLC are described below.
Chemotherapy Followed by Surgery
Neoadjuvant therapy, also referred to as induction therapy, has been applied to the treatment of NSCLC. It entails treating patients with chemotherapy even though there is no clinical evidence that the primary cancer has spread. Lung cancers are particularly attractive targets for neoadjuvant therapy because even though many present as locally advanced disease confined to the chest, patients run a considerable risk of developing distant disease within a short time. Neoadjuvant therapy affords a unique opportunity to assess the sensitivity of the cancer to the drug regimen. This information may be useful in the postoperative period when the possibility of adjuvant therapy is under consideration. Moreover, preoperative neoadjuvant therapy may render resectable a tumor that would otherwise be regarded as unresectable. Another consideration is that the required dose-intensive regimens are apt to be tolerated better before than after surgery. Finally, neoadjuvant therapy may allow for improved drug delivery due to the preserved vasculature of the tumor, and thus may decrease the prospect of developing drug resistance. However, the possibility exists that delaying surgery may be disadvantageous. In patients with locally advanced disease who are at high risk for developing disseminated disease, the delay imposed by the administration of chemotherapy provides an additional period of observation during which a nonresponder may manifest distant disease, thereby precluding surgery.
There have been two phase III randomized trials of neoadjuvant chemotherapy in patients with locally advanced lung cancer (stage IIIA). Some patients had N2 disease alone, while others had T3N0 disease (now considered stage IIB by the 7th edition TNM staging).24,25 In contrast to results in patients with disseminated NSCLC in whom the response to chemotherapy at best approaches 30%, 60% to 70% of patients with locally advanced disease responded favorably. Also, in both trials, the median survival was improved in the group of patients who received neoadjuvant treatment. The explanation for this difference in responsiveness may be the better overall status of patients who are regarded as candidates for surgery and the smaller tumor burden that these patients bear. Alternatively, qualities inherent in the primary tumor that differ from those in the tumor that has metastasized may contribute to a better response to chemotherapy. Currently, there is no way of assessing the response of micrometastatic disease other than the disease-free interval after resection and the OS.
Another experience with neoadjuvant chemotherapy for NSCLC was reported from the Memorial Sloan Kettering Cancer Center.26 The study was prospective but nonrandomized. It included 41 patients with “clinical N2” disease defined as bulky mediastinal adenopathy that could be seen on the conventional chest radiograph or was manifested at bronchoscopy by widening of the carina. Patients received a cisplatin-based regimen plus mitomycin. The overall response rate was 77%; 19 of the patients achieved a complete response that was confirmed by histologic examination. Seventy-five percent of patients were able to undergo resection, even though resectability based on previous experience would have been anticipated to be about 10%. It must be emphasized that these patients had bulky mediastinal lymph node disease, not lymph nodes that appeared grossly normal but in whom disease was subsequently detected. The authors concluded that the results obtained paralleled those noted in neoadjuvant studies with other solid tumors in that response rates to chemotherapy were high, and complete resection rates were high after response to chemotherapy. They identified response to chemotherapy as a significant prognostic indicator for survival.
Concurrent Chemoradiation Followed by Surgery
Various theoretical considerations have led to trials of chemotherapy and radiation followed by surgery (trimodality therapy): (1) Tumor cell subpopulations in locally advanced NSCLC may respond differently to radiation and chemotherapy, and cells resistant to one treatment method may be sensitive to the other; (2) chemotherapy may promote the emergence of radiosensitive cells, thereby increasing the total number of cells killed by continued radiation treatments; and (3) induction of cell cycle synchronization by certain drugs may increase cell killing by radiation and induce recruitment of tumor cells in G0.
SWOG conducted a trial using the cisplatin/etoposide regimen that they had developed with concurrent radiotherapy; the trial included both stage IIIA and IIIB patients.17 All 126 patients underwent mediastinoscopy for histologic evaluation of mediastinal lymph nodes. The response rate to the preoperative therapy was 59%, with 29% having stable disease. The resection rate was 85% in the stage IIIA patients, and 80% in the stage IIIB patients. The 3-year survival was similar in both stage IIIA and IIIB patients at 27% and 24% respectively. The absence of tumor in the mediastinal nodes at the time of surgery was associated with improved survival. Failure was more common in distant, rather than locoregional, sites with relapse occurring in 26 patients.
It remains to be proven that surgery is a necessary part of the treatment of these patients. The high response rate to chemoradiotherapy in the high-performance patients entered into these clinical trials raises the question of whether chemotherapy and radiation might be able to achieve a similar end point with regard to local control. A large intergroup study addressed this question.27 In this trial, 396 patients with stage IIIA pathologic N2 disease were randomized to either concurrent chemotherapy with cisplatin/etoposide and radiation to 4500 cGY followed by surgery or the same chemotherapy regimen with radiation to higher doses of 6100 cGy. The patients who were randomized to surgery had a statistically significant improvement in progression-free survival (12.8 vs. 10.5 months); while there was a trend toward an improvement in OS in the surgery group, this was not statistically significant. In an unplanned subset analysis, patients who seemed to do better were those who had a lobectomy rather than a pneumonectomy, as well as those who had obtained a pathologic response in the mediastinal nodes. Although there was no significant benefit in OS, surgery is still often offered to medically fit patients after induction chemoradiotherapy.
In summary, major issues remain concerning the sequence and type of treatment for locally advanced disease. Although some permutation of combined modality treatment is clearly needed in patients with locally advanced disease, we currently do not have data in the form of randomized control studies that compare the efficacy of the varying combinations of chemotherapy, radiation, and surgery. In patients who can tolerate the added toxicity, combined concurrent chemoradiotherapy is more effective than sequential therapy. Although it has not been proven that surgery after induction chemoradiotherapy confers a survival benefit compared to definitive chemoradiotherapy, surgery is still a preferred approach in many institutions if patients can tolerate trimodality treatment. It remains unclear how combined chemoradiotherapy compares to induction chemotherapy followed by surgery or chemoradiotherapy followed by surgery. At the present time, this is a matter of institutional preference as well as the particular characteristics of individual patients.
METASTATIC NSCLC
Cytotoxic chemotherapy remains the recommended first-line therapy for those with metastatic disease without activating mutations in epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) translocations, and for all patients as second-line therapy. Because the choice of treatment of metastatic NSCLC is based upon the presence of driver mutations, prompt molecular testing of the tumor biopsy material is of increasing importance. Currently the NCCN recommends the testing of all newly diagnosed nonsquamous cell NSCLC (and all cell types in never-smokers) for EGFR mutations and ALK translocations.28 If these molecular targets are not identified, standard first-line therapy is a platinum (cisplatin or carboplatin)-based doublet with consideration for adding bevacizumab, a monoclonal antibody that targets vascular endothelial growth factor (VEGF). In metastatic lung cancer, the goals of chemotherapy are to improve the duration of survival and to palliate the symptoms of disease. The decision to use chemotherapy in the setting of incurable disease is based on patient preference, comorbidities, and PS and requires an ongoing dialogue between the patient and physician throughout the disease course.
 FIRST-LINE CHEMOTHERAPY
FIRST-LINE CHEMOTHERAPY
For patients with adequate PS, the standard first-line chemotherapy recommendations currently consist of a platinum doublet, with several reasonable non-platinum combinations as an alternative. Numerous randomized studies have been conducted that compare the benefits of single-agent versus doublet regimens. A meta-analysis that reviewed 65 of these trials found a significant benefit in response and median survival with a cytotoxic doublet. There was no survival benefit with the addition of a third cytotoxic agent at the time of this analysis. Although there are several cisplatin or carboplatin backbone doublets to consider, carboplatin tends to be favored in the palliative setting due to its better toxicity profile. A large study that was conducted by the ECOG randomized 1207 patients with advanced disease to either cisplatin/paclitaxel, cisplatin/gemcitabine, cisplatin/docetaxel, or carboplatin/paclitaxel.29 The median survival among all four treatment groups was 7.9 months, with a 1-year survival of 33% and a 2-year survival of 11%. There was no clear survival benefit with any one regimen compared with the others. Carboplatin/paclitaxel did seem to have a slightly better toxicity profile. Hence, this is a common regimen in use, but the other doublets are acceptable as well.
Pemetrexed, an antifolate, is a relatively new addition to the available agents in the first-line setting. This cytotoxic agent is unique in that efficacy varies by the histology of the NSCLC and it is labeled by the FDA for use in nonsquamous cell NSCLC only. This labeling is based on a phase III trial in which newly diagnosed patients with advanced NSCLC were randomized to either cisplatin/gemcitabine or cisplatin/pemetrexed in the first-line setting.30 This trial was designed as a noninferiority study and did demonstrate that the doublet cisplatin/pemetrexed was noninferior to a standard doublet with a median OS of 10.3 months. When given with the appropriate folic acid and B12 supplementation, the cisplatin/pemetrexed doublet was associated with less cytopenia and led to a decrease in the need for growth factors and blood transfusions. There was a prespecified histology stratification performed, nonsquamous NSCLC (including adenocarcinoma, large cell carcinoma, and others) versus squamous cell NSCLC. Median OS for the nonsquamous NSCLC histology was 11.0 months for those who received cisplatin/pemetrexed versus 10.1 months for cisplatin/gemcitabine (HR = 0.84, 95% CI: 0.74–0.96). Those with squamous cell histology had a median OS of 9.4 months with cisplatin/pemetrexed versus 10.8 months with cisplatin/gemcitabine (HR = 1.22, 95% CI: 0.99–1.5). As pemetrexed is well tolerated and not associated with alopecia, carboplatin with pemetrexed is a widely used first-line regimen in nonsquamous cell NSCLC.
Two randomized phase III studies have addressed the use of non–platinum-based regimens as first-line treatment. The first, by Kosmidis et al., randomized patients to paclitaxel and gemcitabine versus paclitaxel and carboplatin.31 The study showed similar efficacy with respect to median survival, 1-year survival, and response rate. Both regimens were well tolerated. The largest study to address the role of non-platinum doublets randomly allocated 929 patients to carboplatin/paclitaxel, carboplatin/gemcitabine, or gemcitabine/paclitaxel.32 Again, the results indicated similar efficacy in all three arms. There were differences in toxicities in that anemia and thrombocytopenia were more common in the carboplatin/gemcitabine arm, although peripheral neuropathy and alopecia were more common in the paclitaxel containing groups. For chemotherapy-naive patients, treatment choices tend to be heavily dependent on a patient’s comorbidities and the toxicity profile of each regimen.
The monoclonal antibody bevacizumab, which targets the VEGF, is approved for first-line treatment of NSCLC when combined with cytotoxic chemotherapy. VEGF, a circulating ligand that promotes angiogenesis, is not mutated in NSCLC, and this therapy, though molecularly targeted, is not specific to the tumor. A large randomized phase III study published in 2006 demonstrated an improvement in OS, 12.3 versus 10.3 months (HR = 0.79, p = 0.003) for those patients who received bevacizumab in addition to carboplatin and paclitaxel for advanced NSCLC.33 Due to the high risk of life-threatening hemoptysis seen in early clinical trials, patients with squamous cell lung cancer or a history of hemoptysis were excluded from this study. As an antiangiogenesis agent, bevacizumab has several unique adverse effects including hemoptysis, hypertension, and proteinuria.34 When carefully used in patients with advanced lung cancer, bevacizumab is a novel targeted agent that can improve survival.
 ELDERLY PATIENTS WITH ADVANCED NSCLC
ELDERLY PATIENTS WITH ADVANCED NSCLC
The median age at diagnosis for patients with NSCLC is 65 years; therefore, a significant number of patients diagnosed with advanced disease are elderly (≥70 years). Chemotherapy has been demonstrated to improve survival in the elderly with advanced disease to the same extent as those who are young.35 Although there is an increase in side effects, particularly cytopenias, advanced age is not a contraindication to chemotherapy. Randomized studies dedicated to the treatment of elderly patients with advanced NSCLC are few, but several have provided needed data for this group. In 1999 a randomized study of vinorelbine versus best supportive care in those ≥70 years demonstrated improved survival in those patients who received single-agent chemotherapy.36 A follow-up study compared combined vinorelbine and gemcitabine to either agent alone.37 The combination did not improve outcomes over using single-agent treatment. Due to these findings, national treatment guidelines recommended the use of single agents in the first-line treatment of elderly (70–89 years) with advanced disease. More recently, results of a randomized trial using a carboplatin-based doublet for elderly patients have changed this approach. This study randomized elderly patients to single-agent vinorelbine or gemcitabine versus carboplatin and weekly paclitaxel.38 Those who received the platinum-based doublet had a higher response rate (27% vs. 10.2%, p < 0.0001) and better OS (10.3 vs. 6.2 months, p < 0.001). Higher rates of febrile neutropenia were seen with the doublet therapy. This study supports the use of carboplatin and weekly paclitaxel in elderly patients with good PS with close monitoring for associated cytopenia.
 PATIENTS WITH POOR PERFORMANCE STATUS
PATIENTS WITH POOR PERFORMANCE STATUS
Patients with poor PS represent another common group in need of treatment for advanced lung cancer, but underrepresented in randomized clinical trials. Patients with ECOG PS of 2 – defined as “ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours,” – often are included in first-line trials, although they represent a small proportion of those enrolled. In 2012 data from a randomized clinical trial dedicated to first-line treatment of patients with ECOG PS 2 was presented.39 In this study, patients were randomized to pemetrexed alone versus carboplatin and pemetrexed. Those who received the platinum-based doublet had an improved response rate (24% vs. 20%) and an improvement in median OS (9.1 vs. 5.6 months, p = 0.001). For those patients with ECOG PS 3 – “capable of only limited self-care, confined to bed or chair more than 50% of waking hours” – cytotoxic chemotherapy is of no known benefit, and best supportive care is recommended.
 MOLECULAR TARGETED THERAPY
MOLECULAR TARGETED THERAPY
Advances in molecular biology have enabled the search for novel molecular abnormalities in lung cancer that provide insights into tumorigenesis as well as potential therapeutic targets. The identification of “driver mutations,” those mutations that drive neoplastic transformation and contribute to tumor progression, has provided at least two clinically important targets to date. The epidermal growth factor receptor (EGFR) is a member of the human epidermal growth factor receptor (HER) family, a group of four transmembrane tyrosine kinase receptors expressed on epithelial cells of many organs, including the lung. In response to ligand binding, EGFR (HER1) forms a homodimer with another EGFR molecule or heterodimerizes with a different HER family receptor (HER 2, 3, or 4). This leads to tyrosine phosphorylation on the EGFR intracellular domain and activation of EGFR’s kinase activity, resulting in phosphorylation of target proteins and initiation of downstream signaling. Normal functions of EGFR include epithelial growth and differentiation, cell–cell adhesion, and cell migration.40
Many NSCLCs harbor at least one EGFR mutation. Most EGFR mutations are in the tyrosine kinase domain and result in activation of EGFR and unregulated signaling.41,42 The discovery of these mutations forms the basis for the use of erlotinib and gefitinib, EGFR tyrosine kinase inhibitors (TKI), as a treatment for lung cancer. Certain clinical characteristics are associated with EGFR mutation-positive lung cancer. Mutation-positive cancers are almost exclusively NSCLC, specifically adenocarcinoma.41 EGFR mutations have been reported in 10% to 15% of Western and 25% to 30% of Asian lung cancer patients. EGFR mutations are significantly more common in women and nonsmokers. The presence of EGFR mutations strongly predicts a response to TKI therapy. One pooled analysis of three prior studies demonstrated a response rate to TKI treatment of 81% in mutation-positive cancers compared to <10% in mutation-negative cancers.43 Given the poor response of mutation-negative cancers, therapy selection based on molecular characteristics is superior to using standard clinical criteria. However, further analyses have revealed growing complexity and certain EGFR mutations are associated with resistance to TKI treatment. In addition, over time almost all TKI-responsive lung cancers acquire secondary mutations rendering them resistant to further treatment and relapse is inevitable.44
The presence of driver mutations allows targeting of mutant proteins that are only present in the lung cancer, avoiding systemic toxicity seen in nontargeted therapy. An oral TKI, erlotinib, received FDA approval based on an improvement in OS seen in a randomized phase III trial of erlotinib versus best supportive care in heavily pretreated NSCLC patients.45 The identification of specific activating mutations within EGFR that predict disease response to EGFR inhibitors has made routine genetic testing feasible.46 Multiple randomized clinical trials have demonstrated that patients with activating mutations within EGFR have significant improvement in disease response with decreased toxicity when treated with erlotinib, compared to traditional chemotherapy, as first-line therapy.47 Due to these findings, it is recommended that all patients with newly diagnosed advanced adenocarcinoma of the lung have their cancer tested for EGFR-activating mutations and treated with erlotinib if a mutation is identified.48 The most common toxicities seen with EGFR inhibitors are acneform-type rash, diarrhea, and a small risk of interstitial lung disease (<1% in Caucasian patients). Patients treated with EGFR TKIs develop drug resistance on average about 1 year after treatment.47 Mechanisms of resistance are being studied to provide future targeted therapy in this setting.
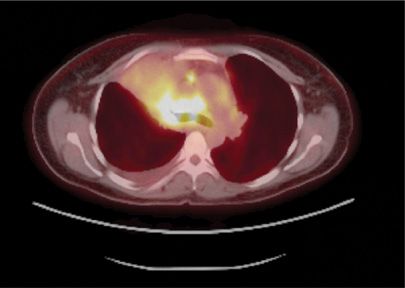
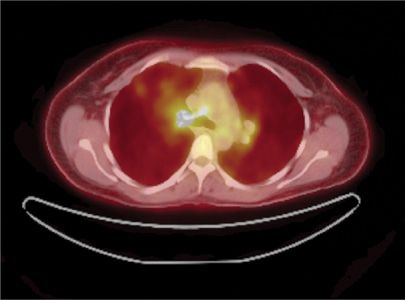
Another clinically relevant molecular subset of NSCLC is the one driven by the newly discovered echinoderm microtubule–associated protein-like 4 (EML4) and ALK translocation.49 The EML4–ALK translocation in NSCLC leads to the constitutive activation of the ALK domain and promotes cell growth and survival. More recently additional fusion partners have been identified with ALK. ALK translocations are found in approximately 2% to 5% of NSCLC, most commonly in never-smokers or light smokers with adenocarcinomas. These translocations are rarely found in tumors that harbor activating mutations in EGFR. A recently reported nonrandomized clinical trial of crizotinib, a TKI that targets the ALK kinase domain, demonstrated encouraging rates of disease control (90%) in patients with advanced lung cancer with EML4–ALK translocations.50 In October 2011, the FDA gave crizotinib approval for treatment of NSCLC with ALK–EML4 translocations (Figs. 114-2 and 114-3).
Figure 114-2 CT scan combined with fluorodeoxyglucose positron emission scan (FDG-PET) of a newly diagnosed metastatic lung adenocarcinoma in a never-smoker. This tumor was found to have an echinoderm microtubule–associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) translocation upon fluorescence in situ hybridization (FISH) analysis.
Figure 114-3 CT/FDG-PET scan of the same patient after 3 months of treatment with crizotinib, an oral tyrosine kinase inhibitor. The imaging shows marked disease response. The patient had progressive disease 9 months after starting crizotinib.
 MAINTENANCE THERAPY
MAINTENANCE THERAPY
Historically, first-line chemotherapy for NSCLC was given for four to six cycles then held, as there was no proven benefit to extending treatment with a doublet past six cycles. If the disease was under control (responded to therapy or stable), all treatment was held and the patient was followed clinically until disease progression. At that time, the patient would be considered for second-line chemotherapy. Over the past few years the availability of drugs that are better tolerated, such as pemetrexed and erlotinib, has enabled investigators to reevaluate this approach and consider maintenance therapy for those with disease control following first-line treatment. Maintenance therapy is given to keep cancer from progressing after response to initial treatment. In NSCLC, maintenance therapy has been divided into two general approaches: Switch maintenance and continuation maintenance. Recent clinical studies have demonstrated an OS benefit with use of maintenance therapy and it has become the standard of care in advanced NSCLC treatment (Table 114-3).