The Athlete and the Cardiovascular System: Introduction
Evaluation and management of the athlete with cardiovascular disease and arrhythmias represents a unique challenge. Although athletes represent symbols of the healthiest segment of our society, they are occasionally affected by cardiovascular conditions that come to the attention of the clinician. The physician can be faced with clinical judgments related to evaluation of symptoms, such as chest discomfort, or signs, such as a murmur, that can be either benign or a manifestation of an underlying cardiac condition. Clinical judgment commonly serves as the basis for recommendations for therapy and athletic participation. In this setting, there is the real risk of failing to detect a cardiac condition, which may result in serious or even life-threatening consequences. There is also considerable risk of unnecessarily treating and restricting sports activity in an athlete misdiagnosed as having an underlying cardiac condition. The consequences of missing an important cardiac diagnosis can be life threatening.
The cardiovascular conditions that predispose to life-threatening complications with athletic activity are now known from pathologic studies.1-3 Recommendations for clinical evaluation, management, and athletic participation are also available to guide clinicians.4,5 This chapter will review cardiovascular disease in the athlete from multiple perspectives. These include distinguishing physiologic cardiovascular adaptations to exercise from true cardiac disease, clinical evaluation of the athlete with suspected cardiovascular disease, arrhythmias in athletes, commotio cordis, guidelines for athletic restriction, and performance-enhancing substances.
The Athlete’s Heart
The athlete’s heart refers to the clinical syndrome of cardiac chamber enlargement, hypertrophy, and normal or augmented ventricular systolic function commonly accompanied by sinus arrhythmia, sinus bradycardia, and a systolic flow murmur (Fig. 101–1).6-10 The notion that the cardiovascular system differentiates physiologically, structurally, and functionally in response to athletic training was initially advanced more than a century ago.6 Using cardiac auscultation and percussion, Henschen6 described enlargement of the heart caused by athletic activity in cross-country skiers. He reported that right and left heart physiologic dilation and hypertrophy resulted from cross-country skiing and that these athletic hearts could perform more work than the heart on a nonathlete.6
Figure 101–1.
Gray area of overlap between athlete’s heart and cardiomyopathies, including myocarditis, hypertrophic cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy. The important diagnostic features compatible with both physiologically based adaptations to athletic training (athlete’s heart) and the pathologic conditions are shown. Reprinted with permission from Maron.1
Debate ensued about whether these adaptations to exercise are physiologic and benign or pathologic and the potential harbinger of disease and disability. The heart of the trained athlete was considered by some to be enlarged and weakened because of the strain of endurance training with potential deterioration of cardiac function and a clinical syndrome of heart failure.8 However, it is now recognized that the athlete’s heart represents a benign increase in cardiac mass, with specific circulatory and cardiac morphologic alterations, representing a physiologic adaptation to systematic training.10 These cardiac changes represent physiologic cardiovascular adaptations to dynamic exercise, also known as isotonic or aerobic training. Thus, the clinical components of the athlete’s heart are most commonly found in athletes participating in endurance or aerobic exercise.
The acute response to training for such athletic activities as cross-country skiing, long-distance running, swimming, or bicycling includes substantial increases in maximum oxygen consumption, cardiac output, stroke volume, and systolic blood pressure, associated with decreased peripheral vascular resistance.10 With several weeks of endurance training, the chronic adaptations to training include increased maximal oxygen uptake from augmented stroke volume and cardiac output and increased arteriovenous oxygen difference. The response to endurance exercise predominantly produces a volume load on the left ventricle.
The morphologic adaptations to endurance training have been quantitatively characterized by multiple studies, largely relying on echocardiography.10-17 The cardiac changes of athletes in response to systematic conditioning are variable, with cardiac remodeling occurring in approximately one-half of trained athletes. These changes include alterations in ventricular chamber dimensions including increased left and right ventricular and left atrial cavity size (and volume) associated with normal systolic and diastolic function.10 Enlargement of the left ventricular (LV) chamber (≥60 mm) occurs in approximately 15% of highly trained athletes.10-13 Occasionally, enlargement of the left ventricle is accompanied by a mild increase in absolute LV wall thickness exceeding upper normal limits (range, 13-15 mm).10-13 Remodeling of LV mass is dynamic and develops after the initiation of vigorous conditioning. Because these changes are reversible with cessation of training, restriction from exercise and reassessment of LV size and wall thickness can be used to distinguish physiologic changes from those associated with hypertrophic cardiomyopathy (HCM).10
Differentiating the physiologic changes resulting from habitual exercise in the athletic heart syndrome with HCM or dilated cardiomyopathy represents a challenge to the clinician (see Fig. 101–1). Physiologic cardiac adaptation from regular exercise leads to an increase in LV wall thickness. This can be difficult to distinguish from pathologic changes of HCM. Criteria favoring HCM include a high degree of LV hypertrophy (wall thickness >16 mm) with an unusual distribution (heterogeneous, asymmetric, or sparing the anterior septum); a small LV cavity (<45 mm); the presence of striking electrocardiogram (ECG) abnormalities; and the persistence of hypertrophy after physical deconditioning. Although many athletes have increased intracavitary dimensions, LV end-diastolic diameter >70 mm is distinctly unusual as a manifestation of the athlete’s heart. LV wall thickness >15 mm is rare even in highly trained athletes. LV wall thickness >16 mm and values above this range raise the possibility of HCM. Hypertrophy (>12 mm) above the normal range is distinctly uncommon in female athletes. Athletes with hypertrophy have increased cavity dimensions, which are not seen in diseases with pathologic wall thickening.11-13
Arrhythmias commonly noted in athletes include sinus arrhythmia, sinus bradycardia, and junctional rhythm. They are frequently accompanied by other manifestations of enhanced parasympathetic tone. Atrioventricular (AV) conduction delays with first-degree and Wenckebach or Mobitz type I second-degree AV block are common in endurance athletes and attributable to enhanced vagal tone and withdrawal of sympathetic tone at rest.18-23 Ambulatory monitoring of athletes has demonstrated ventricular arrhythmias including frequent premature beats, couplets, and nonsustained ventricular tachycardia. These arrhythmias can be within the spectrum of physiologic athlete’s heart.24,25 These arrhythmias are generally not associated with symptoms or an increased risk of sudden cardiac death and are generally reduced with exercise or deconditioning.24,25
A spectrum of abnormal 12-lead ECG patterns are present in up to one-half of trained athletes, more commonly in men and in endurance athletes (Table 101–1).18-23 The most commonly observed alterations include early repolarization patterns, increased QRS voltages, diffuse T wave inversion, and deep Q waves. ECGs in endurance athletes can show mildly increased P wave amplitude suggesting atrial enlargement, incomplete right bundle-branch block, and increased voltages consistent with right ventricular and LV hypertrophy.18-23 Among endurance athletes, voltage criteria for right ventricular hypertrophy are present in a substantial proportion. Abnormal and bizarre ECG patterns suggestive of cardiac disease are noted in a minority of elite athletes.18-23 Most of such ECGs represent only extreme manifestations of physiologic athlete’s heart.
| Diagnosis of Heart Disease | ECG Abnormalities |
|---|---|
| Arrhythmogenic right ventricular dysplasia | T wave inversions anteriorly |
| Epsilon wave | |
| RBBB (complete or incomplete) | |
| Rarely normal | |
| HCM | Left ventricular hypertrophy |
| Pseudoinfarct with Q waves | |
| T wave inversion | |
| Rarely normal | |
| Idiopathic dilated cardiomyopathy | LBBB |
| Prolonged QT | |
| Can be normal | |
| Long QT syndrome | Prolonged QT |
| Abnormal appearance of ST segment | |
| Brugada syndrome | RBBB (complete or incomplete) |
| ST elevation anteriorly | |
| Changes can vary with time | |
| Anomalous coronary artery | Typically no abnormalities |
| Coronary artery disease | Typically no abnormalities |
| Q | |
| ST | |
| Wolff-Parkinson-White | Short PR interval |
| Delta waves | |
| Pseudoinfarct patterns |
Sudden Cardiac Death in the Athlete
The underlying cardiovascular conditions that predispose to the rare and tragic sudden deaths in young athletes are known.1-5,26-29 Available population-based data show that these events occur with an incidence of 2.3 per 100,000 athletes (12-35 years of age) per year.1-5,26-29 The frequency of sudden death in female athletes is lower than in males (2.6 in males vs 1.1 per 100,000 per year in females). Although this predominance of fatal events in male athletes is attributed to the higher participation rate of males in competitive athletics, there is some evidence that male sex can represent itself as a risk factor for sports-related sudden death. Greater prevalence in males of cardiovascular diseases such as cardiomyopathies or premature coronary artery disease potentially resulting in cardiac arrest can contribute to this observation. In athletes younger than 35 years of age, inherited diseases such as HCM, arrhythmogenic right ventricular cardiomyopathy/dysplasia, and congenital coronary artery abnormalities of wrong sinus origin are the most common causes of sudden death (Table 101–2). In athletes older than 35 years, coronary artery disease is the most common cause of sudden death.1-5,26-29
| Structural heart diseases |
| Hypertrophic cardiomyopathy |
| Coronary artery anomalies |
| Left ventricular hypertrophy of indeterminate cause |
| Myocarditis |
| Ruptured aortic aneurysm (Marfan syndrome) |
| Arrhythmogenic right ventricular cardiomyopathy |
| Tunneled (bridged) coronary artery |
| Aortic valve stenosis |
| Atherosclerotic coronary artery disease |
| Dilated cardiomyopathy |
| Myxomatous mitral valve degeneration |
| Asthma (or other pulmonary condition) |
| Heat stroke |
| Channelopathies |
| Long QT syndrome |
| Short QT Syndrome |
| Catecholaminergic polymorphic ventricular tachycardia |
| Brugada syndrome |
| Idiopathic ventricular fibrillation |
HCM is the principal cause of sudden cardiac arrest in athletes in the United States, accounting for up to one-third of sport-related cardiac fatalities (Fig. 101–2).1-5,26-29 HCM is a genetically transmitted disease characterized by genotypic and phenotypic heterogeneity. Usually, the characteristic hypertrophied, nondilated left ventricle with increased wall thickness manifests during adolescence.1-5,26-30 Hypertrophy is usually asymmetric with disproportionate septal thickening and reduction in LV chamber size.1-5,26-29 Decrease in LV compliance can contribute to increased wall stress and inadequate intramural coronary blood filling with exercise. Dynamic LV outflow tract obstruction at rest or with exercise is demonstrable in some patients.
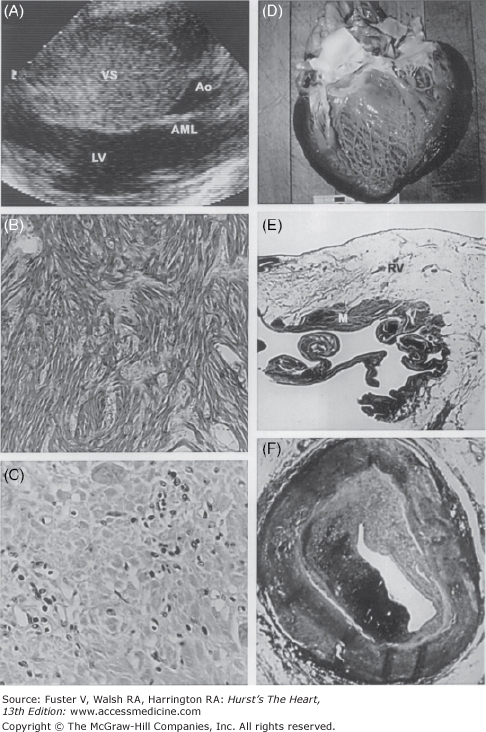
Figure 101–2.
Some cardiac causes of sudden death in young competitive athletes: hypertrophic cardiomyopathy (A and B), myocarditis (C), dilated cardiomyopathy (D), arrhythmogenic right ventricular cardiomyopathy (E), and premature coronary artery disease (F). A. A two-dimensional echocardiogram in the parasternal long-axis view shows extreme asymmetric thickening of the ventricular septum (53 mm) diagnostic of hypertrophic cardiomyopathy. B. Histopathologic analysis shows a substrate of disorganized cardiac muscle cells and a chaotic architectural pattern (hematoxylin and eosin, ×40). C. An area of left ventricular myocardium with clusters of inflammatory mononuclear cells diagnostic of myocarditis (hematoxylin and eosin, ×400). D. A greatly enlarged left ventricular cavity in a patient with dilated cardiomyopathy. E. Arrhythmogenic right ventricular cardiomyopathy with extensive fatty replacement of the wall of the right ventricle adjacent to a small area of residual myocytes (hematoxylin and eosin, ×8). F. A portion of the right coronary artery shows atherosclerotic narrowing and ruptured plaque in a patient with premature coronary artery disease. Ao, aorta; AML, anterior mitral leaflet; LV, left ventricle; M, myocytes; RV, right ventricle; VS, ventricular septum. Reprinted with permission from Maron.1
The characteristic histopathologic marker of HCM is myocardial disarray, with disorganized patterns of myocytes in association with increased interstitial fibrosis and often replacement scarring. These latter fibrotic changes are considered an acquired phenomenon related to microvascular-based myocardial ischemia. This small-vessel disease in HCM involves the intramural coronary arteries, which commonly show dysplasia of the tunica media often with luminal obstruction.1-3,30
Sudden cardiac arrest in athletes with HCM is attributable to ventricular tachyarrhythmias mediated by multiple factors. Myocardial damage observed in athletes dying suddenly supports the notion that acute episodes of silent myocardial ischemia can trigger life-threatening cardiac arrhythmias. Alternate potential mechanisms of cardiac arrest include hemodynamic compromise or primary ventricular arrhythmias.
Arrhythmogenic right ventricular dysplasia is an inherited heart muscle disorder characterized pathologically by fibrofatty replacement of right ventricular myocardium.31-36 It represents the leading cause of sudden death on the athletic field in the Veneto region of Italy, accounting for approximately 25% of cardiovascular sudden death in young competitive athletes.31-36 Clinical manifestations include ECG depolarization and repolarization abnormalities commonly localized to right precordial leads. Cardiac imaging techniques demonstrate right ventricular global or regional morphologic and functional abnormalities. Commonly, premature ventricular contractions or sustained monomorphic ventricular tachycardia with left bundle morphology originate from the right ventricle and are associated with exercise.31-36 Myocardial aneurysms are localized to the posterobasal, apical, and outflow tract regions, resulting in the clinical characterization of these regions as the triangle of dysplasia. Sudden death during physical exercise is likely related to hemodynamic factors, increased right ventricular volume and wall stress, and enhanced sympathetic tone that culminate in ventricular tachycardia.31-36 Physical exercise can acutely increase right ventricular afterload and cavity enlargement, which in turn, can trigger ventricular arrhythmias by stretching the diseased right ventricular musculature.
Anomalies of coronary artery origin can precipitate sudden and unexpected cardiac arrest in athletes probably related to acute ischemia.1-3,37 Most commonly, the anatomic finding at the time of autopsy is the left main coronary artery arising from the right coronary sinus. The acute angle relative to the aorta taken by the anomalous coronary artery leaves a narrowed and compromised lumen, frequently characterized as “slit-like,” which limits coronary blood flow and myocardial perfusion with exercise. Evidence of myocardial ischemia caused by anomalous origin of the coronary artery generally does not manifest on 12-lead resting ECG and is rarely elicited with stress testing. Therefore, false-negative exercise stress tests are common in athletes who have subsequently died suddenly from the above coronary anomaly.37 The diagnosis of anomalous origin of the coronary artery requires a high index of suspicion in young people presenting with exertional chest pain and/or syncope.37 Once diagnosed, anomalous origins of the coronary arteries are generally treated with coronary artery bypass grafting.
Because sudden cardiac death (SCD) in those older than 35 years of age participating in athletic activity most commonly occurs because of atherosclerotic coronary artery disease,1-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree