Advances in diagnostic techniques such as echocardiography, cardiac surgery, and anesthesia, as well as improvement in postoperative management, have undoubtedly contributed to the excellent long-term outcome of patients with tetralogy of Fallot (TOF) following surgical repair. However, these patients present unique challenges because of potential residua and sequelae with increasing duration of follow-up. Right ventricular (RV) outflow tract (RVOT) reconstruction often includes infundibular muscle resection, pulmonary valvotomy or valvectomy, and RV outflow augmentation, and accounts for the most common long-term postoperative complications. Residual or recurrent RVOT obstruction and pulmonary regurgitation (PR) are important determinants of late morbidity, and the most common reason for reoperation. PR is primarily the result of the surgical ventriculotomy as well as extensive infundibulectomy and transannular patching of the RVOT at the time of surgical repair. This can often result in an RVOT aneurysm. Additional factors also contribute to the development and progression of PR, including (a) residual pulmonary valve (PV) abnormalities, (b) pulmonary annulus size, (c) peripheral pulmonary artery stenosis, (d) increased pulmonary vascular resistance, (e) RV diastolic dysfunction, (f) residual atrial and ventricular septal defects, and (g) acquired cardiovascular and pulmonary diseases, including pulmonary hypertension, sleep apnea, systemic hypertension, chronic lung disease, and kyphoscoliosis. Although chronic PR can be tolerated for many years, when left uncorrected, it often leads to progressive RV enlargement and dysfunction, progressive tricuspid valve regurgitation, and eventual right heart failure. PR is also associated with the late development of left ventricular (LV) dysfunction and is recognized as the most important risk factor for atrial and ventricular tachyarrhythmias, as well as sudden death. Assessment of patients with PR following repair of TOF often includes an electrocardiogram, chest radiograph, echocardiogram, exercise testing, cardiac magnetic resonance imaging (MRI), and cardiac catheterization in select patients. This chapter will focus on the echocardiographic assessment of PR following the repair of TOF.
The components of a comprehensive two-dimensional and Doppler transthoracic echocardiographic examination in patients with repaired TOF are summarized in Table 40.1. Each component is essential in the evaluation of patients with TOF to determine outcome and the need for reintervention. In preparation for the echocardiographic examination, it is essential to review the surgical records to obtain an accurate anatomic description of the repair. This ensures adequate understanding of the anatomic variability encountered in these patients and can be very useful as a guide for the echocardiographic examination.

ETIOLOGY AND SEVERITY OF PULMONARY REGURGITATION
PR following repair of TOF is commonly caused by resection of the PV at the time of surgical repair in combination with the concomitant ventriculotomy and transannular patching of the RVOT. These surgical interventions lead to enlargement of the PV annulus and progression to RVOT aneurysm and PR. Alternatively, pulmonary valvotomy, rather than valvectomy, is performed and may result in residual congenital PV abnormalities including stenosis and variable degrees of PR. Bicuspid PV is the most common congenital abnormality of the PV, with a reported incidence of up to 50%, followed by PV dysplasia or hypoplasia. TOF with absent PV occurs in 2% of patients and is associated with severe PR and massive enlargement of the pulmonary arteries. Optimal visualization of the RVOT is needed to assess the etiology and severity of PR. This is accomplished from the parasternal long- and short-axis views of the RVOT. At times, it is possible to image the RVOT from the apical four-chamber or subcostal windows with anterior angulation. These windows are very helpful in the adult patient with emphysema or in patients with poor parasternal windows (Fig. 40.1).
Pulmonary valve regurgitation is characterized by abnormalities in the spectral and color flow Doppler analysis of the RVOT. The severity of PR can be semiquantitatively assessed by echocardiographic techniques detailed in Table 40.2.
Color flow Doppler assessment of PR can be qualitative or quantitative. The two most common qualitative signs of severe PR are the presence of “free PR” and pulsation of the pulmonary arteries. It is not unusual in patients following TOF repair, and especially in those following PV valvectomy, to have “free” PR, whereby color flow Doppler demonstrates unobstructed bidirectional flow across the PV annulus. This severe regurgitation is often associated with vigorous pulsation of the main pulmonary artery and even the PA branches because of lower pulmonary artery diastolic pressure and a wider pulse pressure. The extent of the diastolic color flow signal caused by PR is often used as an indicator of its severity (Fig. 40.2). Brief flow reversal is normal in the branch pulmonary arteries in systole and early diastole because of the pulmonary artery geometry and differential branching of the right and left pulmonary arteries. This normal diastolic flow reversal is very limited in its duration and extent and is not associated with regurgitation into the right ventricle. On the other hand, the presence of persistent retrograde (>50% of the diastolic phase) PR flow in the branch pulmonary arteries that extends into the right ventricle is consistent with severe PR (Fig. 40.2B).
The RVOT area occupied by the regurgitant color flow Doppler signal can be used to assess PR severity. A PR area index, defined as the maximum area of the PR color jet on the parasternal short-axis imaging plane indexed to body surface area, has been shown to correlate well with the PR regurgitant fraction determined by angiography. Kobayashi demonstrated that the PR area index was 0.36 ± 0.29 in grade 1 angiographic PR, compared with 1.48 ± 0.46 in grade 2 and 2.80 ± 0.94 in grade 3 regurgitation. A significant positive linear correlation was observed between the two methods (r = 0.84, p <0.001). However, this technique is limited by two-dimensional image quality, the direction of the PR jet, machine gain settings, and transducer frequency. Given the potential limitations of area measurement, Williams et al. suggested using a linear measurement to assess the severity of PR. The PR jet/annulus ratio is defined as the ratio of the PR color Doppler jet width to the PV annulus dimension in early diastole on the parasternal short-axis view. This ratio has been demonstrated to correlate well with angiographic PR grade. In a study of 26 patients with PR following TOF repair, a PR jet/annulus ratio of 0.4 or less correlated with less than 1+ angiographic PR. On the other hand, a PR jet/annulus ratio of 0.7 separated patients with 2+ from those with 3+ angiographic PR. There was a significant positive correlation between angiographic PR grade and the color jet/annulus ratio (r = 0.95, p <0.001). In the same study, the presence or absence of diastolic flow reversal in the branch pulmonary arteries was assessed using pulsed-wave Doppler and/or color Doppler images in the parasternal short-axis view. Retrograde diastolic flow reversal was present in the branch pulmonary arteries in eight of nine patients with more than 2+ angiographic PR but in no patient who had less than 1+ angiographic PR. The positive and the negative predictive values of retrograde diastolic flow reversal in the branch pulmonary arteries for more than 2+ angiographic PR were 100% and 92%, respectively.
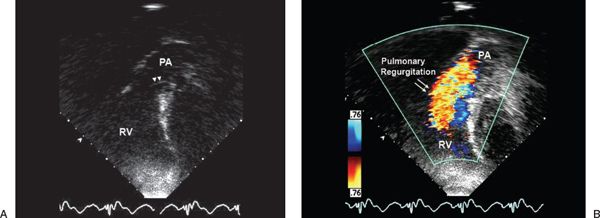
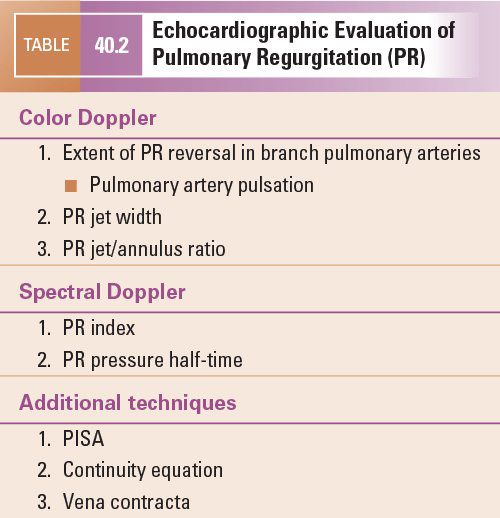
Figure 40.1. Subcostal view of the right ventricular outflow tract. Views without (A) and with (B) color flow Doppler demonstrate the absence of significant outflow tract obstruction (A) but severe pulmonary regurgitation. RV, right ventricle; PA, pulmonary artery.
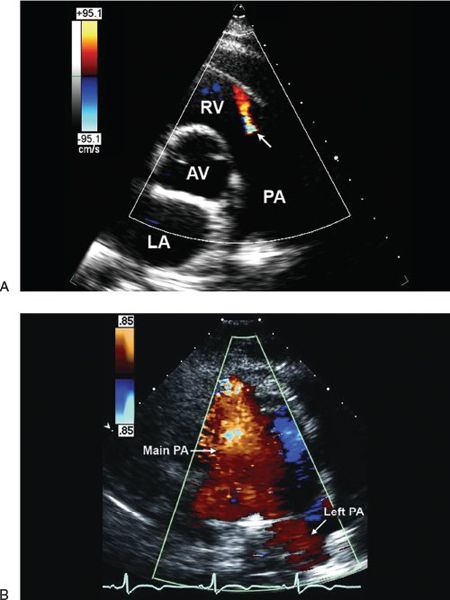
Figure 40.2. Parasternal short-axis view demonstrating diastolic color flow Doppler in the right ventricular (RV) outflow tract. The red jet represents (A) mild and (B) severe pulmonary regurgitation. Notice the difference in the width and extent of the color flow jet that is limited to the RV outflow tract in A but extends from the branch pulmonary arteries in B. AV, aortic valve; LA, left atrium; PA, pulmonary artery; RV, right ventricle.
Other echocardiographic methods, including pulsed-wave and continuous-wave Doppler, have been used for the assessment of PR severity. Spectral Doppler assessment at the level of the PV in patients with PR demonstrates normal forward flow in systole and reversed flow in diastole (Fig. 40.3). In patients with less than severe PR, the diastolic flow reversal is holodiastolic (Fig. 40.3A) and its peak velocity is characteristically less than 1 m/s in the absence of pulmonary hypertension. The finding of a high-velocity regurgitant signal at end-diastole suggests a large difference between pulmonary artery diastolic pressure and RV end-diastolic pressure. On the other hand, in the presence of severe PR, there is early termination of the diastolic flow reversal signal by pulsed- or continuous-wave Doppler assessment (Fig. 40.3B). The regurgitant diastolic velocity peaks early and decreases rapidly as the pressure difference between the pulmonary artery and right ventricle rapidly equilibrates.
This finding is not pathognomonic for severe PR since it is also observed in patients with elevated RV end-diastolic pressure caused by RV diastolic dysfunction, which is not uncommon following TOF repair. However, in the presence of RV diastolic dysfunction, an additional presystolic Doppler forward flow (Fig. 40.4) is often noted. This is the result of end-diastolic RV pressure being higher than the pulmonary artery end-diastolic pressure, leading to forward flow across the PV annulus in late diastole after atrial contraction. This finding helps differentiate RV diastolic dysfunction from isolated severe PR.
Using the continuous-wave PR Doppler signal, further quantification of PR severity can performed by measuring the PV regurgitation index or pressure half-time. Li et al. suggested measuring the ratio of the duration of the continuous-wave PR Doppler signal to total diastolic time (PV regurgitation index) (Fig. 40.5A). This Doppler-derived index correlated closely with cardiac MRI–derived regurgitant fraction (r = –0.82, p <0.01) in a study of 53 consecutive patients with PR following TOF repair. Compared to cardiac MRI, a PR index of less than 0.77 had a 100% sensitivity and 85% specificity for identifying a PR fraction greater than 24%, with a predictive accuracy of 95%. This study also demonstrated that a PR jet width of greater than 0.98 cm had an accuracy of 90% in identifying the group with a PR fraction greater than 24% by MRI. These findings are in agreement with published reports suggesting that severe aortic regurgitation is associated with an LV outflow tract color jet width of greater than 1 cm. Using the same PR velocity profile by continuous-wave Doppler, the pressure half-time can be measured and used for the assessment of PR (Fig. 40.5B). PR pressure half-time was found to be inversely related to PR fraction in the absence of RVOT obstruction and RV diastolic dysfunction. In a prospective study of 34 adult patients with repaired TOF, Silversides et al. demonstrated that the mean pressure half-time measurements were 181 ± 75 ms in patients with a regurgitant fraction of less than 20% by cardiac MRI and 102 ± 29 ms with a regurgitant fraction greater than 40%. A pressure half-time of less than 100 ms demonstrated a high sensitivity and specificity for detecting significant PR.
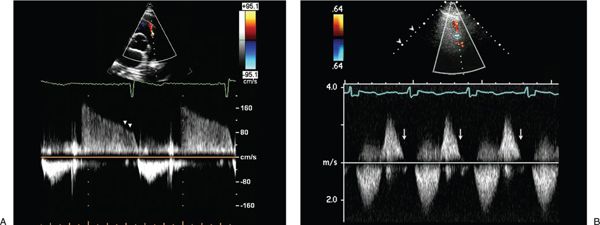
Figure 40.3. Continuous-wave Doppler signal at the level of the pulmonary valve in a patient with mild pulmonary valve regurgitation. A: Normal systolic forward flow and reversed flow that continues throughout diastole, indicating mild pulmonary valve regurgitation. The low end-diastolic velocity suggests normal pulmonary artery diastolic pressure. B: In patients with severe pulmonary regurgitation, the regurgitant diastolic velocity peaks early and decreases rapidly as pulmonary artery and right ventricular pressures rapidly equilibrate leading to early termination of the continuous-wave Doppler signal, with return of the PR Doppler signal to the baseline.
Additional echocardiographic techniques for the assessment of PR severity include (a) measurement of regurgitant volume, fraction, and effective regurgitant orifice by two-dimensional and color Doppler techniques such as the proximal isovelocity surface area (PISA) and the continuity equation, and (b) measurement of the vena contracta. These techniques, however, are not as commonly used in the assessment of PR compared with the assessment of other valvular regurgitation. In addition, each of these techniques has significant limitations. The continuity equation, for example, requires quantitation of the difference between the total forward stroke volume calculated across the PV versus the normal cardiac output calculated across the aortic or tricuspid valve site. Measurement of the PV annular diameter, especially in postoperative patients, has inherent limitations and is subject to significant variability. The presence of tricuspid regurgitation and/or pulmonary stenosis can also affect the accuracy of these measurements. In addition, although PISA appears to be the most reliable method to assess regurgitant volume in patients with mitral and aortic regurgitation, PISA has not gained widespread use in patients with PR because the assumption of a hemispheric shape is not valid in most cases of PR in which flow rates and transorifice pressure gradients are especially low.
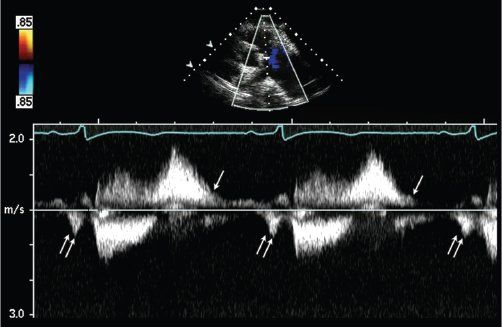
Figure 40.4. Assessment of right ventricular diastolic function in patients with pulmonary regurgitation following tetralogy of Fallot repair. Restrictive physiology leads to antegrade forward flow in the pulmonary artery during atrial contraction (double arrows). This should be noted during all phases of respiration and on five consecutive beats.
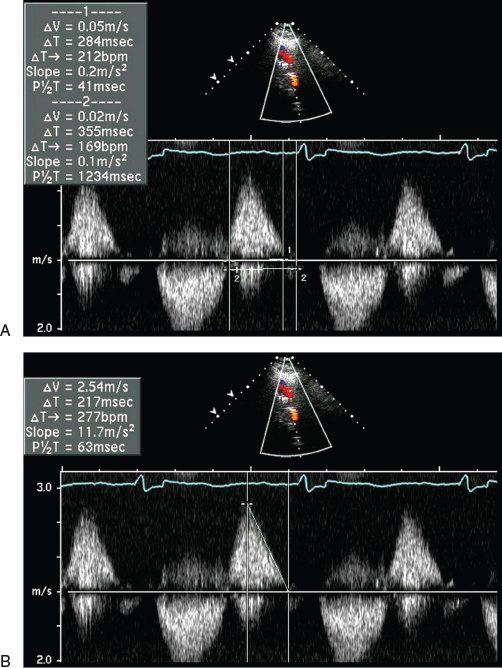
Figure 40.5. Continuous-wave Doppler signal across the right ventricular outflow tract demonstrating measurement of the pulmonary valve (PV) regurgitant index. Ratio of duration of the continuous-wave Doppler pulmonary regurgitation (PR) signal to total diastole (A) and pressure half-time for the assessment of PR severity (B).
RIGHT VENTRICULAR SIZE AND FUNCTION
The right ventricle in patients with TOF is inherently abnormal with significant hypertrophy and fibrosis that persists following surgical repair. Several additional preoperative and postoperative factors are known to contribute to the progressive RV enlargement and dysfunction, including significant tricuspid regurgitation, residual atrial or ventricular level shunts, and residual RVOT obstruction or pulmonary hypertension. RV size and function in repaired TOF are affected by the degree and duration of preoperative cyanosis and pressure overload, as well as factors related to the surgical repair itself, including RV injury secondary to a ventriculotomy, possible coronary artery injury, myocardial injury from inadequate myocardial preservation, and transannular patching within the RVOT. All of these factors are important determinants of the adaptive response of the right ventricle to volume overload from chronic PR. As the right ventricle dilates in response to PR, its ejection fraction initially increases because of the increased ventricular volume. With time, the RV ejection fraction decreases and is a reflection of a decrease in myocardial performance of the volume-loaded right ventricle. Progressive deterioration of myocardial function eventually results in decreased stroke volume with further increases in RV end-systolic and end-diastolic volume. In the current era, every effort is made to maintain PV competence at the time of surgical TOF repair in an attempt to prevent the potential long-term complications related to chronic PR. Current operative techniques often involve a combined transatrial and transpulmonary approach with a very limited RV incision, if needed, for patch augmentation of the RVOT and/or PV annulus. Under these circumstances, the use of a small stiff patch may provide a more superior hemodynamic result than a large expandable pericardial patch. This strategy may avoid significant PR at the expense of mild to moderate residual RVOT obstruction, which is usually well tolerated.
Assessment of RV size and function is a crucial component of the noninvasive evaluation of patients with repaired TOF. Unfortunately, most quantitative two-dimensional echocardiographic measurements of ventricular size and performance are based on the shape of the elliptical left ventricle; these geometric assumptions do not apply to the right ventricle. The right ventricle has a complex geometric shape with thinner walls and abundant coarse trabeculations that make endocardial border delineation challenging. The right ventricle appears to be crescent shaped in cross section and triangular in the lateral view. In addition, the RVOT is muscular and elongated, ending at the PV, which does not have a true bulbar annulus. These differences in ventricular morphology reflect the hemodynamically different roles of the two ventricles. Although the “eye-ball” technique has been commonly used to semiquantitatively assess RV size for many years, the 2010 ASE guidelines clearly state the importance of quantitative assessment of RV size. This however could be challenging in patients with congenital heart disease. Recently, a simple RV linear dimension obtained on transthoracic echo has been suggested as a valid quantitative assessment of RV size in repaired TOF. An RV internal dimension, obtained on parasternal short-axis imaging perpendicular to the mid intraventricular septum of >19.4 mm/m2 in end-systole or >24.5 mm/m2 in end-diastole correlated well with cardiac MR-derived RV end-diastolic volume of >160 cc/m2. Three-dimensional echocardiography promises an accurate determination of RV volume and function. This technique continues to evolve, e.g., with smaller transducers and more improved software.
Given the limitations as a result of RV geometry, many nongeometric Doppler indices have been proposed that use systolic and diastolic time intervals and tissue Doppler imaging to provide indirect information about RV systolic and diastolic function in repaired TOF.
The Doppler assessment of the instantaneous rate of RV pressure increase (dP/dt) can be measured from the tricuspid regurgitant continuous Doppler velocity profile. dP/dt is measured by calculating the rate of RV pressure gradient increase—for example, from 4 mm Hg (1 m/s) to 16 mm Hg (2 m/s). However, this index is sensitive to changes in afterload and does not accurately reflect RV systolic function in patients with residual RVOT obstruction or pulmonary hypertension.
Tissue Doppler imaging quantitates myocardial velocities. For RV assessment, these velocities are measured at the level of the lateral tricuspid valve annulus near the insertion of the anterior tricuspid valve leaflet. These myocardial velocities are a marker of RV systolic and diastolic longitudinal motion. This relatively volume-independent echocardiographic velocity, obtained with or without color Doppler, has been demonstrated to be reduced in patients with repaired TOF compared with controls, and correlates well with reduced RV ejection fraction. Another Doppler-derived quantitative index of global ventricular function is the right-sided myocardial performance index (MPI). This index incorporates both systolic and diastolic parameters and is reported to be a measure of global ventricular function. The RV MPI is measured by dividing the sum of RV isovolumic contraction time and isovolumic relaxation time by the ejection time across the PV. Normal values for the RV MPI have been reported in both children (0.32 ± 0.03) and adults (0.28 ± 0.04). This index has been reported to be a valuable noninvasive method to assess RV function in patients with pulmonary hypertension, as well as Ebstein anomaly and, most recently, in repaired TOF. A study by Abd El Rahman et al. suggested that the MPI is affected by the severity of PR as well as the presence of RV diastolic dysfunction in repaired patients with TOF. All patients with PR had a lower than normal isovolumic relaxation time, while those with severe PR also had a prolongation of isovolumic contraction time compared with patients with mild to moderate PR (103 ± 57 versus 27 ± 41 ms, p
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


