Lung Injury and Repair |
The lung is a major site of exposure to the outside world in air-breathing species. Lung immunity has developed to recognize pathogens and activate appropriate responses, to temper inflammation in response to nonpathogenic exposures and turn off immune responses when danger signals have been eliminated. T lymphocytes play a major role in lung immunity predominantly through the induction of CD4 and CD8 T cells in adaptive immunity. A number of smaller subsets of T lymphocytes also play critical roles in early immune responses to pathogens and stimulating adaptive immune responses. Long-lived memory CD4 and CD8 T cells are crucial for host protection from pathogens, but also may drive chronic disease states, such as asthma. In this chapter the basic biology of T lymphocytes and their relevance in the lung in health and disease will be reviewed.
T LYMPHOCYTE SUBSETS
Lymphocytes make up approximately 10% of leukocytes in the blood and nearly 70% of leukocytes in the normal human lung.1 In both sites, a majority of lymphocytes are T lymphocytes. Lymphocytes include T and B cells and are small, mononuclear cells with a characteristic large nucleus-to-cytoplasm ratio in the resting state. T lymphocytes have their origin in the thymus. T lymphocytes are essential for adaptive immune responses, the type of immunity that develops over a period of days to weeks that fine-tunes an immune response to limit a specific pathogen or insult. The majority of T lymphocytes express alpha-beta (α/β) T cell antigen receptors (TCRs) on the cell surface. The α/β TCR consists of two polypeptide chains with a variable region that binds to antigen, a constant region, and an anchor to the cell membrane. During development in the thymus, T cells undergo TCR gene rearrangement to generate a receptor that has an antigen-binding structure. Each mature T cell bears only this TCR with its unique specificity for antigen binding. Approximately 106 different TCRs develop in an individual, thus allowing the individual to respond to an extraordinary range of antigens throughout life. After exposure to a new antigen, TCRs expressed on T lymphocytes develop even finer antigen-binding capabilities through a process called affinity maturation.
The subsets of T lymphocytes that express receptors with less diversity and ability to recognize antigens include natural killer T (NKT) and mucosal-associated invariant T (MAIT) cells that also express an α/β TCR, and gamma-delta (γ/δ) TCR-expressing cells. These subsets of T lymphocytes are more prominent at sites of pathogen exposure, such as the mucosal surfaces. They express preformed receptors that bind to common pathogen components. This preset ability to bind and react to pathogens allows them to respond quickly, leading to early release of cytokines. NKT, MAIT, and γ/δ T cells are part of the innate immune response to pathogens and other insults that provide signals to initiate and direct CD4 and CD8 T cell activation in the adaptive immune response.
In addition to the α/β TCR, T lymphocytes express coreceptors CD4 or CD8. CD4+ T cells are called T helper (Th) cells because of their ability, through production of cytokines, to stimulate other immune cells. Another subset, CD4 regulatory T cells (Treg), downregulate immune responses. CD8 T cells, traditionally called cytotoxic T cells (Tc), release preformed effector molecules that destroy infected cells. They also produce cytokines that aid in local immunity. TCRs on CD4 T cells recognize peptide antigens that bind to domains on MHC Class II molecules that are present on the surface of antigen-presenting cells (APCs). CD8 T cells recognize endogenously derived antigens bound to MHC Class I molecules. MHC Class I molecules are present on the surface of all nucleated cells, whereas MHC Class II molecules are only present on APCs, including dendritic cells (DCs), B cells, and macrophages. Exogenous protein antigens, such as extracellular pathogens and environmental substances, are taken up by APCs and processed into peptides in endocytic vesicles, which are presented on the cell surface bound to MHC II molecules. Endogenous antigens, such as those of intracellular pathogens, including viruses, are processed and presented by Class I MHC molecules.
 CD4+ T CELLS
CD4+ T CELLS
CD4+ T Cell Activation
Mature, naive CD4+ T cells are released from the thymus into the circulation and enter the lymph node through specialized blood vessels, the high endothelial venules (HEVs). Expression of L-selectin (CD62L) on the naive CD4 T cell along with chemokine signals produced by stromal cells within lymphoid tissues permits attachment and transit through the HEVs into the lymph node.2,3 In the lung, this occurs in the lymph nodes that drain the trachea, bronchi, and lung parenchyma.
Antigens or pathogens that enter the lung are taken up by APCs in the airways and alveoli. DCs are the major APC of the mucosal surfaces. DC in the airway walls and alveoli form a dense network just beneath the epithelium with processes that extend to the airspaces, designed to catch foreign antigens.4 Macrophages in the airways and lungs also engulf large particles and pathogens through phagocytosis, but they are typically poor stimulators of adaptive immune responses, and likely eliminate antigens or induce regulatory responses, rather than activate immunity. After antigen uptake, APCs break down antigens into peptide fragments in endocytic vesicles, where the peptides are loaded onto Class II MHC proteins and transported to the cell surface. Concurrent with antigen presentation, the APC increases expression of costimulatory molecules and migrates to the lung-draining lymph nodes.
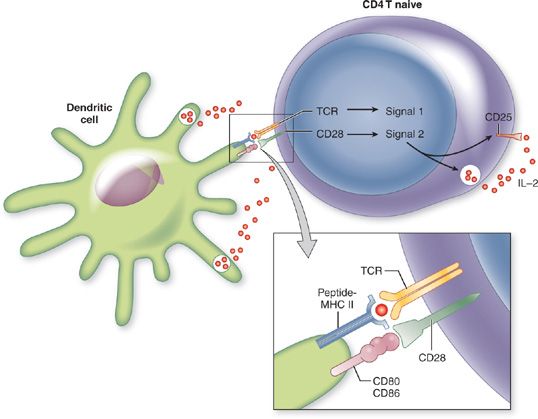
In the T cell area of the lymph node, naive CD4+ T cells move past antigen-expressing APCs, permitting the interaction of TCR and MHC Class II peptide. When the TCR on a CD4+ T cell interacts with its specific antigen on an APC, it ceases to migrate further. T cell activation is initiated upon (1) TCR recognition of MHC class II peptide (signal 1) and (2) costimulatory signaling from the APC (signal 2) (Fig. 25-1). The principal costimulatory molecules expressed on the surface of APCs are CD80 (B7-1) and CD86 (B7-2), which both interact with CD28 on the T cell.5 If both signal 1 and signal 2 are received, the T cell goes into G1 phase of the cell cycle, begins to produce interleukin-2 (IL-2) and undergoes clonal expansion. This gives rise to a population of effector cells with the identical TCR specificity to the parental cell.
Figure 25-1 Two-signal mechanism of CD4 T cell activation. Antigen-presenting cell (APC) takes up a protein antigen and processes it into peptide fragments that are presented by class II major histocompatibility complex (MHC) molecules. Signal 1 required for CD4 T cell activation is recognized by the T cell antigen receptor (TCR) and engagement of class II MHC-peptide complex. Signal 2 is an interaction of CD28 on the T cell with CD80 or CD86 on the APC, termed costimulation. These signals stimulate interleukin-2 (IL-2) production, IL-2 receptor (IL-2R) expression and induce CD4 T cell proliferation.
CD4 Helper T Cell Differentiation
Primary Immune Response Antigen-activated CD4+ T cells differentiate into effector cells of different types. CD4 T cell subsets are defined by the cytokines they secrete, and that pattern of cytokines confers its functional properties (Table 25-1). T helper type 1 (Th1) cells are a subset of CD4+ T cells that secrete the macrophage activating factor, interferon gamma (IFN-γ), and lymphotoxin (LT or TNF-β). T helper type 2 (Th2) cells produce interleukin-4 (IL-4), IL-5, and IL-13. T helper type 17 (Th17) cells produce IL-17A, IL-17F, and IL-22. IL-10, an anti-inflammatory cytokine, was originally defined as a Th2 cytokine, but it is now recognized that it can be synthesized by all Th cell subsets upon appropriate activation.6 Treg produce IL-10 and/or TGF-β1. Since Treg suppress T cell differentiation and APC activation, they are not considered effector cells. Th1 cells stimulate strong cell-mediated immune responses, particularly against intracellular pathogens. Th2 cells, through the production of IL-4 and IL-13, are potent activators of B cell antibody production, particularly immunoglobulin E (IgE). IL-5 secretion by Th2 cells is critical for eosinophil differentiation and maturation. Th2 cells are elicited in immune responses that require a strong humoral component and in antiparasitic responses. Th17 cells stimulate neutrophil mobilization and recruitment, release of antimicrobial peptides, and serve critical host defense functions at mucosal surfaces. An effective immune response to a pathogen commonly results in the induction of a balance of Th1, Th2, and Th17 cells to provide strong cellular and humoral immunity.
TABLE 25-1 T Lymphocyte Subsets and Their Functions
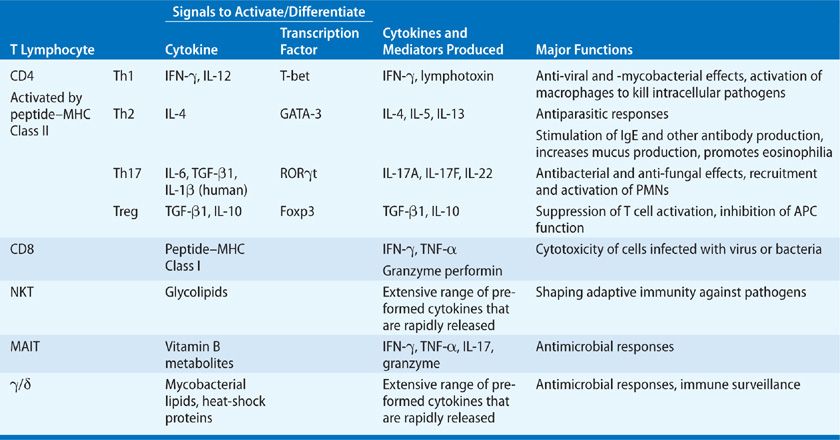
CD4+ Treg comprise subsets of cells generated in the thymus (natural Treg) or induced in the secondary lymphoid tissues (inducible (i) Treg) that produce IL-10 and/or TGF-β1 and express the transcription factor Foxp3. They are often identified by their high expression of CD25 and Foxp3.7 CD4 Treg inhibit the development of Th1, Th2, and Th17 subsets through actions on both APCs, naive T cells, and effector T cells.8 Treg exert their suppressive effects by secreting cytokines, including TGF-β1 and/or IL-10, by cell contact leading to cytotoxicity or metabolic disruption or by suppression of APCs.9
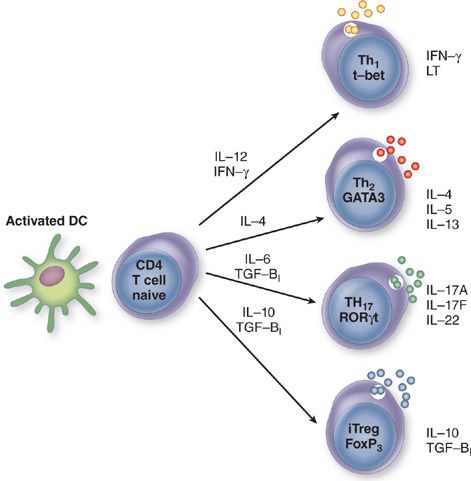
Molecular Mechanisms of CD4 Th Differentiation Differentiation of naive CD4 T cell into Th1, Th2, Th17, or Treg requires the coordinate action of multiple molecular signals induced by stimulation of the TCR, costimulatory molecules, and cytokine receptors (Fig. 25-2). Major factors in these processes are critical lineage-determining molecules important for stimulation of T cell differentiation, simultaneous inhibition of the opposing phenotypes, T cell proliferation, epigenetic remodeling to modify the chromatin structure and cytosine methylation, and expression of key transcription factors.
Figure 25-2 Generation of T helper types 1, 2, and 17 (Th1, Th2, Th17) and regulatory T cells (Treg) from a naive CD4+ T cell. A naive CD4+ T cell secretes very low levels of cytokines. Differentiation along the Th1, Th2, Th17, and Treg pathways is triggered when antigen is presented to the T cell receptor in the context of the major histocompatibility complex (MHC) by the appropriate antigen-presenting cell and a second signal imparted by ligation of costimulatory molecules CD80/CD86 and CD28. Dendritic cells (DCs) represent the key APCs for naive T cells. Cytokines produced in innate immune responses activate DC to produce cytokines that direct differentiation of Th subsets leading to induction of critical lineage–determining molecules, T-bet for Th1 cells, GATA-3 for Th2 cells, RORγt for Th17 cells, and Foxp3 for inducible (i) Treg. The cytokines produced by each subset of CD4 T cells are depicted.
T-bet is one of the lineage-determining molecules required for Th1 differentiation. It belongs to the T-box family of transcription factors that regulate multiple developmental processes. T-bet is upregulated in Th1 cells, through effects of IL-12 and activation of STAT4, and IFN-γ and activation of STAT1.10 Induced expression of T-bet in vitro and in vivo led to IFN-γ production.11 Thus T-bet drives Th1 development. T-bet also promotes Th1 induction through negative regulation of GATA-3.12
The transcription factor GATA-3 is an essential regulator of Th2 differentiation.13,14 Whereas, GATA-3 is expressed at low levels in naive CD4+ T cells,13 it is markedly upregulated in cells differentiating along the Th2 lineage and is downregulated in cells differentiating along the Th1 pathway.13 The differentiation of a naive CD4+ T cell along the Th1 or Th2 pathway is accompanied by extensive reorganization of chromatin structure around the IFN-γ or IL-4/IL-5/IL-13 loci, respectively.15 GATA-3 appears to be the critical downstream regulator of chromatin remodeling around the IL-4 locus.
The orphan nuclear receptor RORγt induces differentiation of naive CD4+ T cells into Th17 cells.16 RORγt is required for the expression of IL-17 and the related gene IL-17F in response to IL-6 and TGF-β in mice or IL-1β in humans.
Cytokines, by stimulating production of critical lineage- determining molecules, are the primary factors that affect CD4 Th generation.17,18 Cytokines produced in innate immune responses modulate how the adaptive immune response will develop. IFN-γ and IL-12 cause induction of Th1 cells. IL-4 and IL-13 drive Th2 cell generation. IL-10 enhances Th2 cell development by inhibiting Th1 cell induction. IL-6, IL-1β, IL-23, and TGF-β1 promote Th17 development.
Other factors that influence CD4 differentiation into Th subsets affect the quality of the signal through the TCR. The dose and structure of the antigen affects the APC–TCR interaction and has been shown to drive Th differentiation along distinct pathways.19 If peptide–MHC Class II complex on the APC has a high-affinity interaction with the TCR, then a Th1-predominant response results, whereas a weaker-affinity interaction leads to a Th2-like response. Very low doses of soluble protein antigen tend to stimulate Th2-predominant responses, and at higher antigen doses, Th1 responses, suggest that the antigen dose affects the APC–T cell interaction. This may have important implications in atopy, since it has been estimated that exposure to common allergens is so small that it does not exceed 1 μg per year. Another line of investigation suggests that high-affinity interactions lead to preferential generation of Th2 cells.20 More recent studies show that the fate of Th differentiation is an effect of both antigen dose and TCR–MHC Class II binding.21 Ultimately, the quality of the signals delivered to the T cell through the TCR influences which Th subsets are activated.
Effector/Memory CD4 T Lymphocytes
It takes 4 to 5 days of proliferation in the lymph node for a naive CD4 T cell to differentiate into an effector cell. Associated with differentiation is a change in expression of cell surface of selectins, integrins, and chemoattractant receptors that permit exit from the lymph node and recruitment to sites of inflammation. With differentiation there is loss of expression of the lymph node homing receptor CD62L and gain of function of tissue homing receptors. Whereas skin and gut effector CD4 T cells each have specific ligands that confer their localization to that tissue through binding to vascular endothelial cells (skin, cutaneous lymphocyte-associated antigen [CLA], small intestine, integrin α4β7), such a lung-specific surface marker has not yet been identified. Human lung effector CD4 T cells are enriched for expression of CCR5, CCR6, chemokine (C-X-C motif) receptor 3 (CXCR3) and the integrins VLA1 (α1β1), CD103 (αEβ7), and VLA4 (α4β1).22 In addition to expression of receptors that respond to specific signals that direct them to the tissue, subsets of effector CD4 Th cells express different panels of chemokine receptors so they can be called to perform specific duties. The type of injury or invading pathogen leads to release of mediators that recruit appropriate, effective populations of CD4 Th cells to manage the specific problem. Th1 cells typically express CCR5 and CXCR3, Th17 cells express CCR6, and Th2 cells express CCR4 and CCR8, and the prostaglandin D2 chemoattractant receptor DP2 (CRTH2) in humans.22
Effector cells that were activated in the lymph node arrive at an inflammatory site in the lung where they proliferate and produce cytokines. The major function of CD4 T cells is to produce cytokines to recruit other inflammatory cells and modulate local host-protective responses. Following this robust response, most activated effector CD4+ T cells die, either by a process of apoptosis or necrosis. A small population of CD4+ cells persists as memory cells for the life of the host.
CD4 memory T cells, upon reexposure to specific antigen, respond quickly with a strong response of longer duration. Subsets of CD4 memory T cells have been identified based on tissue localization and circulation patterns, cell surface markers, and functional differences. Effector memory cells (TEM) are found in blood and in nonlymphoid tissues, such as liver and lung. Central memory T cells (TCM) circulate through secondary lymphoid organs, lymphatics and blood, produce higher levels of IL-2 and proliferate more than TEM.23 The third CD4 memory subset, tissue-resident memory T cells make up large pools of cells in the skin and mucosal tissues, including the lung. TRM remain in the tissue compartment to which they were initially called and do not recirculate. TRM are responsible for barrier protection, exhibit the activation marker CD69 and can be activated locally in the tissue, without trafficking to the lymph node, to provide expedient control of infection.23
CD4 Regulatory T Cells
CD4 Treg comprise subsets of cells generated in the thymus (natural Treg) or induced in the secondary lymphoid tissues (inducible (i) Treg) that produce IL-10 and/or TGF-β1 and exhibit high expression of CD25 and the transcription factor Foxp3. They make up 5% to 10% of the peripheral CD4 T cell population. Cytokines produced by CD4 Treg inhibit the development of Th1, Th2, and Th17 subsets through actions on APCs, naive and effector T cells.8 CD4 Treg are critical for suppressing primary immune responses to self, as indicated by mice and humans deficient in a functional Foxp3 protein that develop autoimmune diseases.24,25 Treg exhibit additional diverse mechanisms to achieve immune suppression in different inflammatory milieu.26
 CD8 T CELLS
CD8 T CELLS
Pathogens that enter the lung activate tissue-resident DCs and migrate to lymph nodes where they activate naive CD8 T cells. CD8 T cells are activated to differentiate into effector cells through interactions with DCs and the provision of two required activation signals, antigenic peptide presented by MHC Class I molecules and costimulatory signals. Viruses and intracellular bacteria are classical activators of CD8 T cells. Cytokines secreted by DC drive the proliferation and differentiation of CD8 T cells to acquire the ability to become effector CD8 T cells that most commonly secrete IFN-γ and TNF-α and have cytotoxic capabilities through production of granzyme B and perforin.27 Effector CD8 T cells may also differentiate into subsets of Tc2 cells that secrete IL-4, IL-5, and IL-13 and Tc17 cells that produce IL-17.28 CD8 memory cells develop from a small subpopulation of cells activated in a primary immune response. Memory CD8, like CD4, T cells include subsets of TCM, TEM, and TRM. While CD8 and CD4 memory T cells may exhibit different homing patterns, memory functions and surface markers appear to mirror observations in CD4 T cells that were described earlier. For example, CD8 TRM, like CD4 TRM, do not recirculate and confer rapid tissue-specific recall responses in the lung.23,29
 T LYMPHOCYTES WITH RESTRICTED TCR DIVERSITY
T LYMPHOCYTES WITH RESTRICTED TCR DIVERSITY
T lymphocytes with less diverse arrays of TCRs accumulate in the lung. They respond to different antigens than traditional α/β CD4 and CD8 T cells. Their numbers in tissues are low, but because many respond to the same antigen, they can be activated quickly without further differentiation. Therefore, they participate in innate immunity and some subsets can expand and adapt to exposures. Thus, these T lymphocyte subsets bridge innate and adaptive immunity. There are two small populations of α/β T cells, iNKT cells and MAIT cells that have semi-invariant α chains. Thus, they have undergone somatic mutation in the thymus to generate a TCR, but they have limited diversity due to fewer possible TCR combinations. Both iNKT and MAIT cells are found in high frequencies in mucosal tissues.
Natural Killer T Cells
Type I or invariant (i)NKT cells are a small subpopulation of α/β T cells that express a narrow repertoire of TCRs due to invariant expression of the TCR variable (V)α chain and limited TCR Vβ chains. In humans Vα24-Jα18 pairs almost exclusively with Vβ11, whereas in mice Vα14-Jα18 pair with a limited number of Vβ chains. iNKT cells respond to glycolipid antigens presented on the MHC-like molecule CD-1d, which is a member of a family of nonpolymorphic proteins that bind to lipids, rather than peptide antigens, and is expressed on populations of DCs, B cells, and macrophages.30
iNKT cells leave the thymus upon maturation and migrate into tissues. In mice, iNKT cells are enriched in the liver and spleen. In humans iNKT cells are highly enriched in the omentum but have not been well characterized in all tissues.31 In the bronchoalveolar lavage (BAL) of normal control subjects iNKT cells constituted less than 1% of the lymphocyte population.32 In mouse, iNKT cells may be 5% to 10% of the lung lymphocytes.33 Murine studies show that iNKT cells are resident in the lung and, for the most part, do not recirculate to other organs.34 iNKT cells also reside in the lung microvasculature in the resting state and upon exposure to inhaled lipids, become activated and extravasate into the lung parenchyma where they contribute to lung inflammation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree