Systemic Pulmonary Shunting
Because most congenital heart defects are managed by total correction, shunting procedures are now performed in select patient populations. Systemic to pulmonary artery shunts offer excellent palliation in patients with anatomically complex cardiac anomalies, in whom definitive repair is best delayed. They are also indicated as a source of controlled pulmonary blood flow in the initial management of neonates with single-ventricle anatomy.
A common application of the systemic to pulmonary artery shunt is in the neonate with a ductal-dependent pulmonary circulation. The ability to keep the ductus arteriosus patent with an infusion of prostaglandin E1 allows these patients to be stabilized and undergo surgery on a semiurgent basis in an unhurried manner.
Types of Shunts
The Blalock-Taussig shunt was introduced in 1945. Classically, it consists of anastomosing the subclavian artery to the pulmonary artery on the side opposite the aortic arch. However, with some technical modifications the subclavian artery can be anastomosed to the pulmonary artery on the same side as the aortic arch.
Other shunting procedures were subsequently introduced. They include the Potts shunt (descending aorta to the left pulmonary artery), Waterston shunt (ascending aorta to the right pulmonary artery), central shunt (interposing a graft between the ascending aorta and the main pulmonary artery), and the modified Blalock-Taussig shunt (interposing a GORE-TEX tube graft between the subclavian or innominate artery and the right or left pulmonary artery).
The Potts shunt was abandoned because it was cumbersome to perform, difficult to close, and could cause high flow and the early development of pulmonary vascular disease. The Waterston shunt lost favor because of the high incidence of injury to the pulmonary artery and the difficulty in controlling the amount of flow through the shunt. The classical Blalock-Taussig shunt is rarely used. Currently, most surgeons perform a central shunt or modified Blalock-Taussig shunt through a median sternotomy. The relative disadvantage of this approach requiring a redo sternotomy and dissection of adhesions for the next procedure is outweighed by the superior exposure and ability to place the patient on cardiopulmonary bypass should hemodynamic instability occur. If the patient requires a second shunt procedure before definitive repair or as further palliation, a modified Blalock-Taussig shunt through a thoracotomy approach may be indicated.
Modified Blalock-Taussig Shunt with GORE-TEX Tube Graft Interposition
Interposition of a GORE-TEX tube graft between the subclavian or innominate artery and the right or left pulmonary artery is the most commonly performed shunt procedure. Currently, most shunts are performed through a median sternotomy as an isolated procedure or as part of a palliative surgery for single ventricle anatomy. Under some circumstances, a thoracotomy approach is indicated. With either approach, it should be remembered that the lumen of the subclavian or innominate artery is the limiting factor to the volume of flow. In neonates, a 3.5- or 4-mm graft is used; for older infants, a 5-mm graft is usually selected.
Median Sternotomy Approach
This approach has several advantages. The pulmonary end of the shunt can be placed more centrally, potentially allowing better and more uniform growth of both pulmonary arteries. The ductus arteriosus can be occluded at the conclusion of the procedure preventing excessive pulmonary circulation in the early postoperative period. The ductus arteriosus can be ligated when a left thoracotomy approach is used but can rarely be accessed through a right thoracotomy. Finally, if the patient becomes unstable, cardiopulmonary bypass can be quickly initiated through a median sternotomy.
Technique
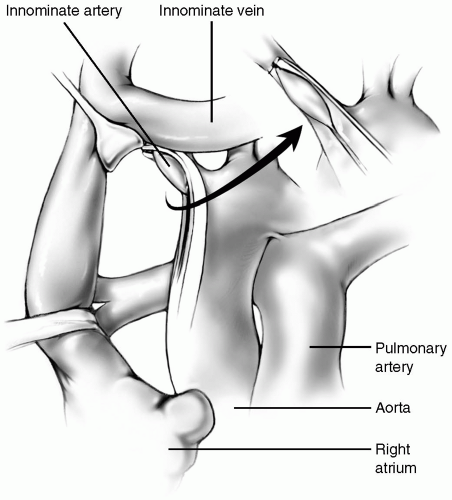
After opening the pericardium, traction sutures are placed on the pericardial edges. The aorta and pulmonary arteries are dissected free using scissors or electrocautery on a low setting. Downward traction on the main pulmonary artery allows the ductus arteriosus to be identified and encircled with a tie or cleaned free of surrounding tissues in preparation for later metal clip closure. The innominate artery is dissected to allow application of a C-clamp. The right pulmonary artery is then dissected away from the posterior aspects of the ascending aorta and superior vena cava. It is mobilized circumferentially and the right upper lobe branch is identified.
If the shunt is being performed without cardiopulmonary bypass, systemic heparinization (1 mg/kg) is administered just before the clamp is applied to the innominate artery.
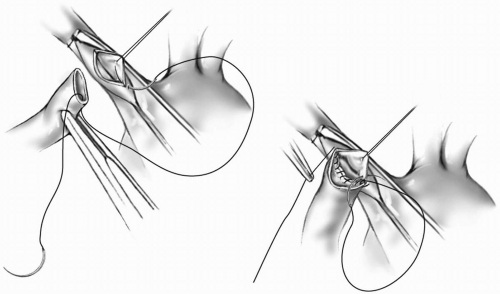
The GORE-TEX graft is trimmed obliquely. A fine vascular C-clamp is applied to the innominate artery so that the inferior aspect of the artery is centered in the excluded portion (Fig. 18-1). The handle of the clamp is then raised to position the inferior edge of the innominate artery anteriorly. A longitudinal incision is made in the artery, and a fine adventitial suture is placed on the superior edge of the arteriotomy to keep the lumen open. The anastomosis is started near the heel with 7-0 or 8-0 Prolene suture. With one end of the suture tagged, the other needle is passed from the inside to the outside of the graft at the heel. The suture is pulled through to the half-way point, then the same needle is passed from outside to inside the artery at the heel. The inferior suture line is completed using the same needle continuing one to two bites beyond the toe and tagging the suture line outside the graft (Fig. 18-2). The other needle is passed from inside the artery at the heel, and the suturing is continued until both suture lines meet. The sutures are tied together.
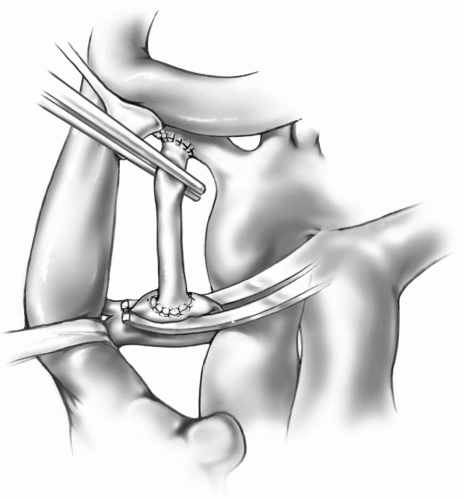
With the other end of the graft occluded, the vascular clamp on the innominate artery is carefully removed and the anastomosis is checked for leaks. The length of the GORE-TEX graft is measured to just reach the superior aspect of the proximal right pulmonary artery. The graft is divided transversely at this site after placing a fine straight vascular clamp on the graft just below the innominate anastomosis. Alternatively, the C-clamp may be reapplied to the innominate artery to occlude inflow to the graft. The right pulmonary artery is grasped with a fine C-clamp so that the superior aspect is in the middle of the clamp. The clamp is then rotated so that a longitudinal incision can be made on the superior edge of the pulmonary artery. The arterial opening should be approximately two-third of the diameter of the graft lumen as the pulmonary artery stretches. A double-armed, 7-0 or 8-0 Prolene suture is passed from inside to outside of the pulmonary arteriotomy at the 12 o’clock position. The other needle is tagged, leaving one-half of the suture pulled through. The first needle is then passed from outside to inside on the graft. The superior portion of the anastomosis is completed in this way, carrying the same needle two bites past the 6 o’clock position. The other needle is then passed from inside to outside on the graft, and the suturing is continued until the two sutures meet (Fig. 18-3). The C-clamp on the pulmonary artery is now removed, and the sutures are tied together. The clamp on the graft is removed, and a check for leaks is done. A thrill should be appreciated by palpating the graft.
The median sternotomy approach allows the pulmonary artery end of the shunt to be placed as centrally as possible. The aorta must be mobilized and retracted leftward with a traction suture on the right side of the aorta, a vein retractor, or the back of the C-clamp itself (Fig. 18-3).
Care must be taken when applying traction to the aorta to prevent compression or kinking of the coronary arteries. If any electrocardiographic changes are noted or hemodynamic instability occurs, the traction suture, retractor, or clamp must be repositioned immediately.
When the shunt is opened and flow through it confirmed, the ductus arteriosus, if present, should be occluded to prevent pulmonary overcirculation. Too much pulmonary blood flow may lead to systemic hypoperfusion and an inadequate diastolic blood pressure resulting in coronary ischemia.
Before incising the pulmonary artery, hemodynamic stability and systemic oxygenation with the C-clamp in place should be assessed. The clamp may interfere with ductal flow, and reapplying it more distally on the right pulmonary artery may rectify the problem. However, if desaturation or hemodynamic compromise persists after repositioning the clamp, the patient should be placed on cardiopulmonary bypass for support during this anastomosis.
Tension on the anastomosis owing to too short a tube graft may cause suture line bleeding and an upward pull on the pulmonary artery, which can lead to distortion or stenosis of the proximal right pulmonary artery. A graft that is too long may kink, thereby compromising flow through the graft.
At the completion of the procedure, the pericardium is loosely reapproximated with three or four interrupted sutures. A small chest tube is placed in the anterior mediastinum before a standard sternotomy closure is performed.
Modified Left Blalock-Taussig Shunt
It may be preferable to place an interposition GORE-TEX tube graft between the subclavian and pulmonary arteries
through a thoracotomy incision. This may apply when a child requires a second shunt procedure before definitive repair or palliation. In this case, a left thoracotomy approach is generally used. Some surgeons prefer a thoracotomy approach for the initial shunt. In this case, a right-sided shunt may be used because it is easier to take down. The technique is essentially the same for both sides. The following description pertains specifically to the left side.
through a thoracotomy incision. This may apply when a child requires a second shunt procedure before definitive repair or palliation. In this case, a left thoracotomy approach is generally used. Some surgeons prefer a thoracotomy approach for the initial shunt. In this case, a right-sided shunt may be used because it is easier to take down. The technique is essentially the same for both sides. The following description pertains specifically to the left side.
Incision
A left thoracotomy through the fourth intercostal space provides satisfactory exposure.
Technique
The left lung is retracted inferiorly and posteriorly, and the local anatomy is evaluated. The left pulmonary artery is identified. The parietal pleura overlying it is incised, and the artery is mobilized medially toward the pericardium and distally to the hilum of the lung.
Sometimes the exact identity of the vessels within the hilum of the lung may not be clear. If there is any doubt as to the exact location of the pulmonary artery, it can be traced from within the pericardium through a short longitudinal incision on the pericardium, just anterior and parallel to the left phrenic nerve.