Introduction
Syncope is a symptom in which transient loss of consciousness (TLOC) occurs as a consequence of a spontaneously self-limited period of cerebral hypoperfusion.1–3 A wide range of conditions may be responsible for triggering syncope; in many instances the faint is relatively benign (e.g. vasovagal faint) but others (e.g. certain cardiac arrhythmias) may have more serious implications. In any case, whether the underlying problem is “innocent” or potentially life-threatening, syncope may lead to physical injury, accidents that put others at risk, and economic loss. Consequently, the management goals are to identify the specific causes(s) of the symptoms, and thereafter develop a treatment plan designed to prevent recurrences.1
This chapter provides a brief summary of the major causes of syncope, including nomenclature concerns, but focuses principally on clinical management (diagnosis and treatment), particularly changing treatment concepts for neurally mediated reflex syncope and orthostatic syncope. Whenever possible, the recommendations provided here are adapted from the European Society of Cardiology Syncope Task Force guidelines.1 However, that was not always possible. In such cases, we have either elected not to provide a “level” of recommendation or, when it seemed reasonable, to indicate by means of an asterisk (*) that the recommendation is based on the authors’ opinion.
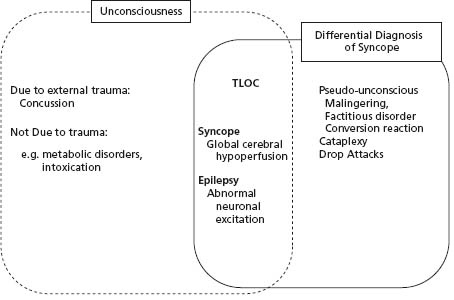
“Syncope” must be distinguished from the many other forms of transient loss of consciousness (TLOC) or “TLOC/ syncope mimics” (Fig. 42.1). Unfortunately, physicians remain uncertain regarding this distinction. Despite the efforts of some,1–4 the confusion continues to be exacerbated by imprecise writing in the medical literature.5–7 For example, the recent scientific statement from the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) confuses the definition of “true syncope” with the broader concept of TLOC.5 Similar imprecise characterization of syncope may be found in a widely cited report from the Framingham Study6 and in a relatively recent clinical review article in the British Medical Journal.7 Inaccurate advice from seemingly authoritative sources unnecessarily confounds the “syncope” evaluation and results in the undertaking of expensive but low-yield tests such as electroencephalograms (EEGs) and imaging studies of the head. In an effort to clarify matters, several groups have attempted to both address the vagaries of terminology1–4 and develop more cost-effective ways to evaluate syncope patients.1,8–14
Figure 42.1 Schematic illustrating the relationship of “syncope” to other conditions that cause real or apparent transient loss of consciousness (TLOC). Syncope is characterized by causing TLOC as a result of a self-limited inadequacy of cerebral perfusion.
Incidence, natural history, and prognosis
Frequency in the population
Syncope has been variably reported to account for approximately 1–3% of emergency department (ED) visits (the 1% is the more recent estimate) and 1–6% of general hospital admissions in the United States.15–17 In numerical terms, the National Hospital Ambulatory Medical Care Survey of 2006 in the United States noted that primary diagnoses of “syncope and collapse” (ICD-9; 780.2) had increased from approximately 887 000 ED visits in 2001 to greater than 1 125 000 ED visits in 2006. On the other hand, “syncope and collapse” listed among all US hospital discharge diagnoses in the 2006 National Hospital Discharge Survey remained relatively constant over this same time period: 405 000 in 2001 and 411 000 in 2006. This suggests, perhaps, that ED physicians are becoming less hesitant to have such patients evaluated on an outpatient basis.
In part, variations among published reports examining epidemiologic aspects of “syncope” may relate to the terminology issues alluded to above. In any event, as pointed out by Sheldon and Serletis,18 conventional clinical epidemiologic terms such as “prevalence” (the proportion of people with the disease) and “incidence” (the proportion of people acquiring the disease in a sampling interval) are not readily applicable to the problem of understanding the epidemiology of “syncope”. For instance, these authors argue quite insightfully that syncope prevalence must approach zero since at any point in time very few people are unconscious. Similarly, since syncope is a transient symptom that vanishes, its true “incidence” is difficult to assess. Sheldon and Serletis18 suggest more meaningful indices such as cumulative proportion, cumulative event rate or cumulative incidence.
Given the limitations noted above, it is possible to obtain from the literature a rough sense of the “burden” of syncope in various population subsets. Thus, the Framingham Study (in which biennial examinations were carried out over a 26-year period in 5209 free-living individuals) reported occurrence of at least one syncope event in approximately 3% of men and 3.5% of women in a relatively broad-based free-living population sample.19 The first syncope occurred at an average age of 52 years (range 17–78 years) for men and 50 years (range 13–87 years) for women. Further, while syncope occurred at virtually all ages, syncope burden tended to increase with advancing age from 8/1000 person-exams in the 35–44 year old age group to approximately 40/1000 person-exams in the ≥ 75 year age group.
In more selected populations, syncope has been estimated to occur in 15% of children < 18 years of age, 25% of the young military population, and in up to 23% of a nursing home population > 70 years of age.15,16,18–24 However, with respect to the elderly patient, the reported frequency may well be an underestimate since up to 20% of these individuals are believed to be forgetful for such episodes (i.e. retrograde amnesia).
Two reports provide the most up-to-date assessment of syncope burden among free-living persons. Ganzeboom et al22 surveyed medical students in The Netherlands and found that 39% had fainted at least once by about age 25 (women 47% versus men 24%). A Canadian report indicated that the likelihood of at least one faint was 37% by age 60, and almost all first spells occurred by age 40.24 Combined, these studies suggest that 40% of people faint at least once in their lives.
Recurrence rates
Among patients who have experienced syncope, it has been estimated that syncope recurrences occur in about 30%.19,22,24 About one-third of patients have recurrences of syncope by 3 years of follow-up, with most recurrences occurring within the first 2 years. Predictors of recurrence of syncope include:
- age <45 years
- a psychiatric diagnosis, and
- a long history of prior syncope recurrences; in particular, patients with more than six syncope spells and a positive tilt-table test (i.e. suggestive that syncope is of neurally mediated reflex origin) have a high risk of syncope recurrence (>50% over 2 years).
Mortality
The presence and severity of co-existing structural heart disease are the most important predictors of mortality risk in syncope patients. Thus, among individuals with cardiac syncope (i.e. primary cardiac arrhythmia, an ischemic episode or severe valvular heart disease), the 1-year mortality is high (ranging between 18% and 33%) compared to that for patients with either non-cardiac (including “vaso-vagal”) causes of syncope (0–12%) or unexplained syncope. The risk of death differences are even more striking when considering “sudden cardiac death” events: the 1-year incidence of sudden death is approximately 24% in patients with a cardiac cause versus about 3% in the other two groups.6,19
Although patients with cardiac syncope have higher mortality rates compared with those of non-cardiac or unknown causes, cardiac syncope patients do not as a rule appear to exhibit a higher mortality when compared with matched controls having similar degrees of heart disease.25–29 There are, however, some important exceptions to this rule. These include severe aortic stenosis (average survival without valve replacement of 2 years), hypertrophic cardiomyopathy in which syncope at diagnosis is a predictor of increased sudden death risk, and possibly patients with heart failure and severe left ventricular dysfunction.29 The mortality risk associated with syncope in the setting of one of the channelopathies (e.g. Brugada syndrome, long QT syndrome (LQTS)) or in the presence of arrhythmogenic right ventricular dysplasia (ARVD) may reasonably be considered to fall into this exception as well, if the syncope is known to be due to ventricular tachyarrhythmias.
A number of subgroups of syncope patients can be identified who have an excellent prognosis. These include young healthy individuals without heart disease and normal electrocardiogram (ECG), individuals with the most common forms of neurally mediated reflex syndromes (i.e. vasovagal faint, most situational faints) and syncope of unknown cause (5% first-year mortality). Patients with carotid sinus syndrome (CSS) may be expected to have a more worrisome prognosis given the close association of CSS with underlying atherosclerotic cardiovascular disease. However, syncope may result in injury. Major morbidity such as fractures and motor vehicle accidents are reported in 6% of patients, and minor injury such as laceration and bruises in 29%. Recurrent syncope is associated with fractures and soft tissue injury in approximately 12% of patients.
Prognostic issues remaining to be resolved
Failing to distinguish the additional risks associated with recurrent syncope from the risks accompanying any underlying co-morbidity (especially heart disease) is a common error. The result is that physicians may overlook the need to address syncope risk directly as they focus on the treatment of the underlying heart disease (or occasionally vice versa). For instance, implantable cardioverter-defibrillator (ICD) therapy is often recommended in patients with syncope and left ventricular ejection fraction (LVEF) <30%. Clearly, an ICD may well be indicated, but it would be indicated even in the absence of syncope. On the other hand, while the ICD may prevent sudden death, it takes time to detect an arrhythmia and initiate treatment; consequently, it may not protect the patient from injury due to recurrent syncope (even if the syncope is due to ventricular tachyarrhythmias). Further assessment of the patient is needed.
The impact of syncope has been a particular concern in “channelopathy” patients, specifically in LQTS or Brugada pattern.30–33 With regard to LQTS, in one large prospective observational trial in >800 patients,30 cardiovascular end-points including apparent syncope, cardiac arrest and sudden death occurred in 23% of patients. Syncope was associated with a fivefold increased risk of cardiac arrest or sudden death, but it was not a sensitive predictor of death risk. Similarly, in patients with the so-called Brugada pattern on ECG who have a history of syncope, the observation has been made that syncope is not a sensitive predictor of or risk factor for sudden death. In a multicenter study, 40% of 220 Brugada patients implanted with an ICD had a history of syncope, but the patients with syncope were not at a higher risk of appropriate ICD discharge than those who had been asymptomatic.31 Similarly, in a large meta-analysis encompassing 1140 patients (262 of them (23%) with a history of syncope), the patients with syncope had the same risk of ventricular tachyarrhythmias as those who had been without syncope, and a significantly lower risk than those presenting with documented cardiac arrest.33
The syncope victim is usually in the upright position at the onset of the attack. Syncope in the supine position should raise the possibility of marked bradycardia or very rapid tachyarrhythmias. Supine syncope due to neural reflex hypotension has also been reported, but is believed to be uncommon.
The relationship of upright posture to increased susceptibility to transient cerebral hypoperfusion is readily understandable since in humans, upright posture substantially reduces the safety factor for cerebral perfusion. Upon arising from being seated or supine, approximately 500–1000 mL of blood moves relatively rapidly from the central circulation to the venous system below the diaphragm. Physiologic adaptive countermeasures, including peripheral vaso-and venoconstriction, enhanced muscle pump activity, thoracic respiratory pump action, and moderate tachycardia, automatically come into play to prevent an otherwise inevitable drop in cerebral blood flow. Additionally, the “autoregulatory” nature of the cerebrovascular circulation helps to maintain flow despite fluctuation of perfusion pressure. Thus, the healthy well-hydrated human is usually able to cope quite effectively with even very abrupt positional change. On the other hand, despite built-in compensatory mechanisms, many individuals (even those in good health) may experience transient symptomatic hypotension when moving quickly from the supine or seated to upright posture. In the vast majority, this scenario is typically abrupt in onset, occurs promptly after posture change (i.e. “immediate” hypotension), is brief in duration, and is manifest only as a temporary loss of vision (so-called “gray-out”).34 The immediate hypotensive event is rarely a cause for concern. However, of a more serious nature, in some patients frank syncope may occur after movement to upright posture (so-called postural or “orthostatic” faints). In these cases, the faint is usually somewhat delayed after the postural change, suggesting that the mechanism is more than just the “immediate” hypotensive response.35 In some of these patients there appears to be a more complete failure of physiologic adaptation mechanisms, due to diseases of the autonomic nervous system. In the majority of cases, however, more commonly encountered conditions are the cause, including:
- drug therapy (e.g. diuretics, vasodilators)
- generalized frailty in a chronically ill individual
- dehydration
- recent exposure to extended periods of bedrest or gravitation-reduced environment.
Quite often, hypotension leading to syncope is the result of neurally mediated reflex disturbances of blood pressure control (e.g. vasovagal faint, situational faints, carotid sinus syndrome, etc.). Vasovagal and situational faints (e.g. postmicturition syncope) are among the most frequent causes of syncope in all age groups, and are the most common of the so-called neurally mediated reflex syncope syndrome (Box 42.1). In some of these cases the predominant outcome of inappropriate neural reflex activity is parasympathetic-induced bradycardia or asystole (so-called cardio-inhibitory syncope). In others, symptomatic hypotension is due primarily to inappropriate vasodilation (i.e. vasodepressor syncope). In most cases, however, both phenomena contribute.1,36,37
Primary cardiac arrhythmias are a less frequent cause of syncope in the general population than are the conditions noted above. Nonetheless, when at fault (usually in individuals with structural heart disease) they are important to identify since they may have a worrisome prognosis, and they can usually be treated effectively whether by drugs, ablation or implantable devices.
Paroxysmal supraventricular and ventricular tachycardias (SVT, VT), intermittent atrioventricular (AV) block or sinus node pauses are among the most common primary cardiac arrhythmias associated with syncope. However, the basis for syncope in the setting of these arrhythmias may be complex. By way of example, an abrupt blood pressure decrease starting immediately after tachyarrhythmia onset occurs even in patients with normal hearts. Certainly, the rapid heart rate plays an important role in this phenomenon. However, other factors may contribute. Sudden atrial distension and vigorous atrial contraction against closed AV valves (in tachycardias with AV dissociation) may cause not only a reduced forward flow but also a neurally mediated reflex vasodilation that may undermine the effectiveness of adaptive vaso- and venoconstrictive mechanisms. Further, in elderly or infirm patients, muscle pump and respiratory pump activity may be compromised, thereby increasing the chance that perfusion pressure may fall below the autoregulation capability of the cerebrovascular bed.
In patients with cardiac disease such as hypertrophic cardiomyopathy (HCM) or aortic stenosis, both SVT and VT may cause cardiac syncope. However, neurally mediated reflex hypotension may also contribute; the triggers are believed to come from the heart itself, presumably via excessive stretch on ventricular and atrial mechanoreceptors.
The European Society Task Force on Syncope guidelines,1 the American College of Cardiology Task Force report on tilt-table testing,38 the American Academy of Emergency Medicine practice guidelines39 and the ACC/AHA Scientific Statement on Syncope5 provide recommendations regarding the appropriate strategy for diagnosis and treatment of syncope (Fig. 42.2), including risk-stratification advice regarding the need for hospitalization. For the most part these recommendations have been primarily “expert” consensus-based documents. However, recently several observational studies and randomized clinical trials have begun to contribute to our better understanding of how syncope management practices might be improved.
Figure 42.2 Algorithm, modified from that proposed by the European Society of Cardiology Syncope Task Force guidelines,1 depicting an approach to the clinical evaluation of syncope. As discussed in the text, a careful initial evaluation by an experienced clinician can often provide a “certain” diagnosis of the cause of syncope. In such cases, no further evaluation is needed, and treatment as needed may begin. However, in many instances the outcome of the initial assessment is only a “suspected” diagnosis or even an “unexplained” cause. In such cases, further selective testing is needed. An appropriate pathway is indicated here. See text for details. ILR, implantable or insertable loop recorder; NMS, neurally mediated reflex syncope; OH, orthostatic hypotension; TLOC, transient loss of consciousness.

Classification of the causes of syncope
Box 42.1 provides a classification of the principal causes of syncope likely to be encountered in general internal medicine or family practice. However, it should be borne in mind that a single diagnosis may not be sufficient; more than one cause may contribute to the clinical picture (especially in the older patient).
Neurally mediated reflex syncope
The vasovagal (or “common”) and situational faints occur in virtually all age groups, and may be triggered by any of a variety of factors, including prolonged standing (particularly in warm, emotionally charged environments), unpleasant sights, and pain. The diagnosis is most often suspected from the medical history; however, the history is not always definitive, especially in the elderly.40 In such cases, the accounts of eye-witnesses become crucial and tilt-table testing (Class IIa, Level C) and use of implantable loop recorders (Class I, Level B) become important diagnostic tests.1,41–45 The ATP test, although controversial (Class IIb *, Level C), may also prove helpful to the clinician, especially in the older patient in whom pacemaker therapy is a consideration.46–49
BOX 42.1 Syncope and syncope mimics: a classification
Syncope
- Neurally mediated reflex syncope
- Vasovagal faint
- Carotid sinus syncope
- Situational faints (e.g. cough syncope, post micturition, defecation, etc.)
- Pain triggered
- Orthostatic syncope
- Chronic blood/plasma loss (e.g. hemorrhage, diarrhea)
- Primary autonomic failure syndromes (e.g. pure autonomic failure, multiple system atrophy, Parkinson’s disease with autonomic failure)
- Secondary autonomic failure syndromes (neuropathies: diabetic, alcohol, amyloid)
- Drugs (vasodilators, diuretics)
- Primary cardiac arrhythmias
- Sinus node dysfunction (including bradycardia/tachycardia syndrome)
- AV conduction system disease
- Paroxysmal supraventricular and ventricular tachycardias
- Channelopathies (LQTS, Brugada, etc.)
- Implanted device (pacemaker, ICD) malfunction
- Structural cardiovascular or cardiopulmonary disease
- Severe aortic stenosis
- Acute myocardial infarction/ischemia
- Cardiac valvular disease/ischemia infarction
- Atrial myxoma, ball-valve thrombus
- Obstructive cardiomyopathy
- Pericardial disease/tamponade
- Prosthetic valve malfunction
- Pulmonary embolus
- Pulmonary hypertension
- Subclavian steal syndrome
- Cerebrovascular
- Vascular steal syndromes
- Acute hypoxemia
- Migraine
- Syncope mimics – disorders resembling syncope
- Seizures
- Psychogenic “syncope” (conversion reaction)
- Hyperventilation (hypocapnia)
- Intoxications
- Accidental falls (usually in elderly/infirm persons)
Carotid sinus syndrome is one form of neurally mediated reflex syncope that tends to occur in older male patients, probably related to higher predilection to atherosclerotic cardiovascular disease in that population. Published experience suggests that CSS may be a more important cause of non-accidental “falls” or “spells” in older individuals than had previously been appreciated.50 The condition is suspected by careful history taking, but its presence is confirmed when symptoms are reproduced during carotid sinus massage (best undertaken with the patient in the upright position secured on a tilt-table), particularly if there is concomitant induction of an asystolic pause, paroxysmal AV block, and/or a marked drop in systemic arterial pressure (Class I, Level B). In the absence of symptom reproduction, a pause of ≥ 5 seconds is often considered sufficient to support the diagnosis (assuming other etiologies of syncope have been excluded). An implantable loop recorder (ILR) may also prove helpful in suggesting the diagnosis of CSS, but limitations include absence of direct correlation of syncope with carotid stimulation, and the possibility of “missing” a major vasodepressor hypotension episode since it is not yet possible to monitor systemic pressure with these devices.
Orthostatic (postural) syncope
Frank syncope induced by moving from supine or seated to upright posture is an important problem in elderly or less physically fit individuals, or patients who are volume depleted. Iatrogenic factors such as excessive diuresis or aggressive prescription of antihypertensive drugs may be important contributors. Inadequate fluid intake, especially by older persons who have diminished thirst drive or who purposely avoid fluids due to genitourinary system issues, is another important contributor. Less often, primary forms of autonomic nervous system dysfunction are the underlying predisposing cause, including pure autonomic failure, multiple system atrophy, and Parkinson’ s disease.51–53
In most clinical practices overt primary autonomic dysfunction is relatively rare. Nevertheless, becoming familiar with its presentations may be of value in determining the cause of orthostatic symptoms in some patients, and perhaps better understanding symptoms in a much larger group of patients who have less well-defined disorders, such as postural orthostatic tachycardia syndrome (POTS). Furthermore, as the features of autonomic dysfunction become more widely appreciated, subtly affected patients will be identified more often. For instance, Low et al54 reviewed an experience in 155 patients referred for assessment of suspected orthostatic hypotension. Findings in this referral population revealed that among the most severely affected symptomatic patients (n = 90, mean age 64 years), pure autonomic failure accounted for 33%, multisystem atrophy for 26%, and autonomic/diabetic neuropathy for 31%. Finally, secondary autonomic dysfunction due to neuropathies associated with chronic diseases (e.g. diabetes mellitus), toxic agents (e.g. alcohol) or infections (e.g. Guil-lain–Barr é syndrome) are relatively common in medical practice, and may also cause syncope in association with orthostatic hypotension.
Primary cardiac arrhythmias
Primary cardiac arrhythmias (i.e. those rhythm disturbances arising as a result of cardiac conduction system disturbances, anomalous electrical connections, myocardial disease or “channelopathies”) are less frequent causes of syncope than either the neurally mediated or orthostatic triggers, but they are of importance due to their more worrying prognostic implications. In general terms, the arrhythmias most often associated with syncope or near-syncope are the bradyarrhythmias accompanying sinus node dysfunction or AV block, and the tachyarrhythmias of ventricular origin. Only a very brief overview is provided here as these conditions are discussed in detail elsewhere in this volume.
Sinus node dysfunction
Sinus node dysfunction (SND) comprises various sinus node and/or atrial arrhythmias that may result in persistent or intermittent periods of inappropriate slow (sinus bradycardia, sinus pauses, sinoatrial exit block) or, on the other hand, excessively fast heart beating (most often atrial fibrillation or atrial flutter), or both manifestations at different times in the same individual.55,56 SND may be considered to be “intrinsic” or “extrinsic”. Intrinsic SND is closely associated with structural disturbances in the atria (e.g. fibrosis, chamber enlargement) resulting from aging, disease or prior cardiac surgery. Extrinsic SND may be due to autonomic nervous system influences, cardioactive drugs, and/or metabolic disturbances.57–59 In terms of syncope, bradyarrhythmias associated with SND appear to be the more important culprits than tachyarrhythmias.56
Disturbances of atrioventricular conduction
Disturbances of AV conduction range from relatively innocent abnormalities (e.g. first-degree AV block) to complete conduction failure (third-degree AV block).60–62 As a rule, however, it is the more severe forms of acquired AV block (that is, Mobitz type II block, “high-grade” and complete AV block) that are most closely associated with syncope. In contrast to acquired forms of AV block, congenital complete AV block was for a long time considered to be relatively benign. However, it is now known that syncope is more common and mortality greater in congenital AV block patients than had previously been suspected.60
Whether syncope occurs in patients with various forms of bundle branch block or fascicular conduction system disease depends both on the risk of developing high-grade or complete AV block and on the risk of occurrence of ventricular tachyarrhythmias.62 Diagnostic electrophysiologic testing may be useful in such cases.
Ventricular tachyarrhythmias
Ventricular tachyarrhythmias (VT) have been reported to be responsible for syncope in up to 20% of patients referred for electrophysiologic assessment. Risk factors favoring VT as a cause of syncope include underlying structural heart disease, underlying conduction system disease, congenital or drug-induced LQTS or other so-called “channelopathies”. The latter conditions present special risks of both syncope and sudden death.63–68 Syncope in channelopathy patients may be due to any cause (including vasovagal faints), but the specific and most critical risk is syncope due to polymorphic VT, including torsades de pointes. The mere presence of a “history of syncope” is not sufficient reason to implicate a VT as the cause and consequently, while ICD therapy in such a situation (i.e. no evidence of a VT) is recommended by guidelines (Class IIb, Level B), there continues to be room for debate.68 In any case, the assumption that VT is the cause of syncope should not be taken lightly even if an ICD has been placed. An ICD may prevent premature death, but may act too late to prevent syncope (or, as alluded to already, may not even be relevant to the cause of syncope in a particular individual).25,28,29
Supraventricular tachyarrhythmias
Supraventricular tachycardias are relatively infrequent causes of syncope.1,69,70 Tachycardia rate, the volume status and posture of the patient at time of onset of the arrhythmia, the presence of associated structural cardiopulmonary disease, the integrity of reflex peripheral vascular compensation, and the promptness with which normal cardiac activity resumes after termination of the tachycardia are key factors determining whether hypotension of sufficient severity to cause syncope occurs.70
Structural cardiovascular or cardiopulmonary disease
Structural cardiac or cardiopulmonary disease is often present in syncope patients, particularly those in older age groups. However, in these cases it is usually arrhythmias associated with structural disease, rather than the abnormal structure itself, that are more often the cause of symptoms. In terms of syncope directly attributable to structural disease, the most common is that which occurs in conjunction with acute myocardial ischemia or infarction, pulmonary embolism and pulmonary hypertension. The basis of syncope in these conditions is multifactorial, including both the hemodynamic impact of the specific lesion as well as neurally mediated reflex effects.
Syncope may also occur and be a presenting feature in conditions in which there is fixed or dynamic obstruction to left ventricular outflow (e.g. aortic stenosis, hypertrophic obstructive cardiomyopathy (HOCM)). In such cases symptoms are often provoked by physical exertion, but may also develop if an otherwise benign arrhythmia should occur (e.g. atrial fibrillation). The basis for the faint is in part inadequate blood flow due to the mechanical obstruction. However, especially in the case of valvular aortic stenosis, ventricular mechanoreceptor-mediated bradycardia and vasodilation is thought to be an important contributor. In obstructive cardiomyopathy, neural reflex mechanisms may also play a role in causing syncope, but atrial tachyarrhythmias (particularly atrial fibrillation) or ventricular tachycardia (even at relatively modest rates) can also trigger hypotension.71–74
Cerebrovascular, neurologic, and psychiatric disturbances
Cerebrovascular disease and neurologic disturbances are rarely the cause of true syncope.1 However, these conditions may result in a clinical picture that can be mistaken for syncope even by experienced practitioners. For example, temporal lobe epilepsy may closely mimic (or induce) neurally mediated reflex bradycardia and hypotension. In such cases, neurologic consultation becomes essential.
Fixed cerebrovascular disease is almost never responsible for syncope due to the redundancy of the cerebral circulation (multiple vessels accessing the circle of Willis).75 Consequently, searching for carotid artery disease in syncope patients by Doppler studies or angiography is rarely useful. On the other hand, global cerebrovascular spasm (possibly as part of a migraine syndrome) could trigger syncope. However, as well as can be determined, the frequency with which this mechanism occurs is vanishingly small; most faints in “migraineurs” are of vasovagal origin.75
Psychiatric conditions do not cause syncope, but may result in syncope mimics. Thus, conversion reactions may be responsible for symptoms that are difficult to distinguish from loss of consciousness events. Similarly, anxiety attacks may be associated with severe hyperventilation resulting in hypocapnia and transient alkalosis that cause “syncope-like” symptoms. Considerable effort (including multiple consultations to various specialists) is often expended before a pseudo-syncope diagnosis is comfortably established. When these conditions are suspected, psychiatric consultation is needed.
Strategy for the diagnostic evaluation
Structure of the syncope evaluation
The ultimate goal of the diagnostic evaluation is to establish a sufficiently strong correlation between symptoms and detected abnormalities to permit both an assessment of prognosis and initiation of an appropriate treatment plan. Care must be taken to avoid assuming that the mere presence of an abnormality provides a basis for the patient’s symptoms.
A careful history and physical examination form the basis for the initial assessment of the syncope patient (see Fig. 42.2). Alone, these first steps provide a diagnosis in a substantial proportion of patients, especially in younger individuals in whom vasovagal faints predominate and the history is clear-cut. However, in many other cases even an experienced history-taker cannot be certain of the diagnosis, and further testing is needed. In this regard, the front-line physician must determine whether such testing requires hospitalization or if outpatient evaluation is safe (i.e. minimal chance of mortality or syncope recurrence with either injury or necessitating hospitalization within the next few weeks to months). Thus, patients who may be safely evaluated outside hospital include those “low-risk” individuals who exhibit any of the following features: absence of evident structural heart disease and a normal ECG, history of recurrent syncope over many years or suspicion that the presentation is that of a “syncope mimic” (e.g. psychogenic pseudo-syncope). Unfortunately, however, apart from these very low-risk patients, it is difficult to “risk stratify” moderate- to high-risk TLOC/ syncope patients.76,77
Although, as noted earlier, ED practice patterns in the US may be changing, hospital admission for syncope management remains very frequent despite the fact that in many instances outpatient assessment would be just as safe and far less expensive. Potentially, greater availability of “syncope management units” (SMU), “rapid access blackout clinics” (best illustrated by that in Manchester, UK) or “TLOC/syncope clinics” may reduce inappropriate hospital admissions.
Medical history taking
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


