Surgical Treatment for Heart Failure
The armamentarium for treating end-stage heart failure has evolved significantly in recent years. Although cardiac transplantation remains the gold standard treatment for this disease, the increasing disease prevalence and limited donor organ supply limit the applicability of this strategy as an across-the-board solution. Several different medical therapies have been established through prospective randomized trials as a means for improving ventricular function, symptoms, and clinical outcomes. These include the use of β-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and aldosterone antagonists.1 Biventricular pacing has emerged as a method for improving symptoms and survival and limiting complications in patients with heart failure and cardiac dyssynchrony.2 However, these treatments are all limited in their efficacy, with mortality rates as high as 18% at 6 months.3 Accordingly, surgical management of heart failure continues to represent one of the fastest growing aspects of adult cardiothoracic surgery.
The heart failure state may be the result of ischemic changes causing myocardial dysfunction (and thus dilatation and often valvular dysfunction) or may begin first with ventricular chamber distension and remodeling causing increased wall tension and subendocardial ischemia. Thus, apart from ventricular assist devices (described later), the major surgical approaches to heart failure can be categorized by their mode of therapeutic benefit: (1) coronary revascularization, (2) repair of valvular defects resulting in pressure and volume overload, and (3) anatomic ventricular restoration. In all cases, it is imperative to determine preoperatively whether the given patient is also a transplantation candidate, so as to prepare for the possible eventuality of postcardiotomy assist device bridging to transplantation.
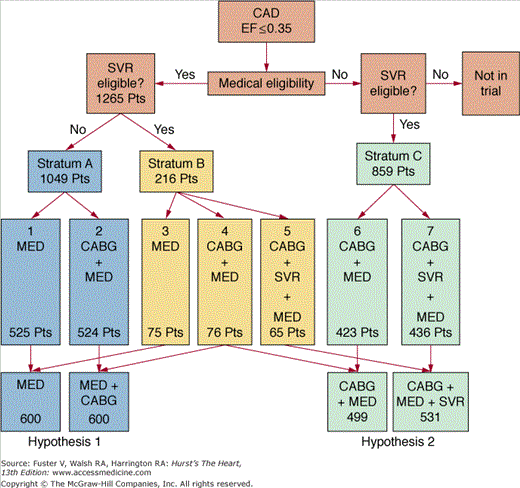
Although several nonrandomized studies have documented the utility of various surgical approaches to heart failure, there is currently no Level 1 evidence to establish the role of coronary artery bypass graft (CABG) w ventricular remodeling procedures in these patients. The Surgical Treatment for Ischemic Heart Failure (STICH) trial was designed specifically to address these questions.4 In this ongoing trial, patients were included if they had an ejection fraction (EF) ≤35%, coronary anatomy suitable for revascularization, and age ≥18 years. Exclusion criteria included the need for an aortic valve procedure, cardiogenic shock or intra-aortic balloon pump support, percutaneous coronary intervention planned for coronary artery disease treatment, acute myocardial infarction as the cause of left ventricle (LV) dysfunction, more than one prior CABG, and noncardiac illness that either imposed a substantial operative mortality or was associated with a life expectancy of <3 years. Based on these selection criteria, patients were randomized according to the protocol illustrated in Fig. 30–1. Patients were treated with medical therapy, CABG, or CABG with surgical ventricular restoration (SVR). The study was designed to address two separate hypotheses:
FIGURE 30–1.
Randomization on the Surgical Treatment for Ischemic Heart Failure (STICH) trial. CABG, coronary artery bypass graft; CAD, coronary artery disease; EF, ejection fraction; MED, medical therapy; SVR, surgical ventricular restoration. Reproduced with permission from Joyce et al.4
- Hypothesis 1 (coronary revascularization hypothesis): Improvement in myocardial perfusion by CABG combined with intensive medical therapy improves long-term survival compared with medical therapy alone.
- Hypothesis 2 (LV reconstruction hypothesis): In patients with anterior LV akinesia, LV shape and size optimization by SVR combined with CABG and medical therapy improves long-term survival free of cardiac hospitalization compared with CABG and medical therapy without SVR.
High-Risk Revascularization
Coronary revascularization has been shown to benefit patients with depressed LV function in numerous studies. Improvements in survival, quality of life, and LV function have been demonstrated in patients with an EF of <30%.5 With hospital mortality rates of <5%, this approach to the heart failure population rivals outcomes for transplantation in selected patients. Revascularization has traditionally been pursued when the recruitment of ischemic, but viable, myocardium is felt to offset the risk of (re)operation. However, randomization in STICH did not exclude patients from the CABG arm of the study based on preoperative viability studies. The techniques of surgical revascularization of the patient with end-stage heart failure do not differ significantly from those in other patients. Some surgeons prefer the use of warm-induction cardioplegia based on the presumption of improved myocardial preservation. In contrast, off-pump coronary bypass grafting can be particularly challenging in these patients because of the large heart size and often concomitant valvular regurgitation, which may presage hemodynamic instability with positioning and stabilization of the heart, particularly for left circumflex coronary artery revascularization.
Mitral Valve Procedures
Mitral valve dysfunction in end-stage heart disease may be the result of degenerative valvular damage, ischemic mitral insufficiency, or a combination of both. Ischemic mitral insufficiency affects both the valvular and subvalvular apparatus by causing ventricular dilatation and papillary muscle ischemia. Thus, in the early stages of ischemic mitral regurgitation, myocardial revascularization may be sufficient to reverse the pathologic process. If permanent damage has been sustained or if irreversible ventricular/annular dilatation has occurred, some form of mitral valve repair or replacement is necessary.
Bolling et al6 and Bishay et al7 have demonstrated success with the use of a substantially downsized mitral annuloplasty ring to correct ischemic mitral regurgitation in patients with dilated cardiomyopathy. Reduction of central valvular regurgitation from annular dilatation in this setting is intuitively appealing and also represents the basis on which emerging technology has based several minimally invasive techniques. For example, splint-type devices placed through the coronary sinus or transmyocardially have been tested in animals and have reduced the size of the mitral annulus. The Coapsys device (Myocor, Maple Grove, Minn) represents such a device in which a single, flexible cord is placed from the anterior septum to the lateral wall and shortened to allow for better coaptation of the valve leaflets while potentially avoiding the use of cardiopulmonary bypass.8 Other devices under investigation use catheter-based platforms to reduce mitral valve regurgitation through the same type of annular reduction procedure or through an edge-to-edge approach.
If mitral valve repair is not possible and significant mitral insufficiency persists intraoperatively, it is essential that mitral valve replacement be performed, preferably with preservation of the subchordal attachments to help maintain ventricular geometry and function.9-11
Ventricular Restoration/Remodeling
The techniques of ventricular restoration evolved out of the early experiences with ventricular aneurysm resection—a complication that occurs with much less frequency in the current era of coronary reperfusion. Described initially in 1996, the Batista procedure of partial left ventriculotomy involves resection of LV muscle (not scar tissue) between the anterior and posterior papillary muscles (the territory of the first marginal branch of the circumflex coronary artery) in conjunction with mitral valve repair in an effort to restore ventricular geometry.12 Although the Batista procedure is now essentially obsolete, its somewhat radical concept of ventricular excision to restore ventricular elliptical geometry has been applied in related techniques, especially those to treat LV aneurysms.13
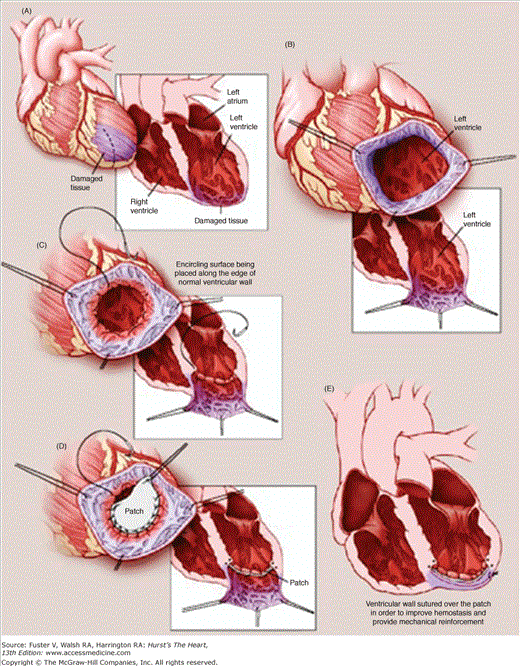
LV aneurysms are characterized by anatomic thinning of the ventricular wall from transmural myocardial infarction, with resultant paradoxical motion of the aneurysmal segment, resulting in volume overloading and distension of the already impaired LV.14 Early surgical approaches, first described by Cooley et al,15 involved linear closure of the aneurysmal segment and demonstrated only marginally acceptable outcomes. This technique failed to address the distortions of ventricular geometry associated with LV aneurysm disease, and both the Jatene and Dor techniques were established to address these additional concerns. In the Jatene technique, the aneurysm is resected, and the aneurysmal portion of the septum is imbricated, thereby stabilizing it against normally contracting myocardium.16 In the Dor technique, the aneurysmal segment is not resected but rather is excluded from the ventricle with a Dacron patch after a purse-string suture is placed at the base of the aneurysmal segment (Fig. 30–2).17 Although the Dor procedure has been reported with a <10% early mortality and 5- and 10-year survival rates of 65% and 80%, respectively, controversy remains about the technical aspects of the operation and their impact on outcomes.18
An international group of investigators, referred to as the Reconstructive Endoventricular Surgery Returning Torsion Original Radius Elliptical Shape to the Left Ventricle (RESTORE) investigators, assembled to review the role of anterior ventricular restoration in the treatment of postinfarction ventricular dilatation. Results from the RESTORE registry demonstrated improved ventricular function and functional status in 1198 post–anterior infarction patients with chronic heart failure who were treated with surgical anterior ventricular restoration (SAVR) in addition to concomitant CABG (95%), mitral valve repair (22%), or mitral valve replacement (1%). In this registry, 5-year survival was 69%, and 5-year freedom from hospital readmission for congestive heart failure was 78%.19 These favorable results promoted enthusiasm for a randomized comparison of SAVR with medical therapy or with coronary artery revascularization, thus prompting the design and execution of hypothesis 2 in the STICH trial.
The LV reconstruction arm of the STICH trial compared patients undergoing revascularization with patients undergoing revascularization combined with additional surgical restorative procedures. Patients were selected for the LV reconstruction arm of the study if they demonstrated an LV end-systolic volume index ≥60 mL/m2. A total of 1000 patients with an EF <35%, coronary artery disease amenable to CABG, and predominantly anterior LV dysfunction were enrolled; 499 patients underwent CABG alone, whereas 510 patients also underwent reconstructive procedures (SAVR). With a median follow-up time of 48 months, there was no difference detected in the primary end points (death from any cause and hospitalization for cardiac causes) despite the fact that patients who underwent SAVR experienced a 19% reduction in end-systolic volume index compared with only a 6% reduction in the CABG arm (Fig. 30–3).20 These findings have led many to conclude that SAVR does not have a role in the routine management of patients with LV dysfunction who are scheduled for revascularization.21 Critics of this study contend that the results should be interpreted with caution because many patients who were candidates for reconstruction may have been withheld from the study based on a perception by the treating surgeon that the procedure would be beneficial in these cases. Indeed, the investigators contend that a broad range of positions of equipoise may have confounded the randomization.20
FIGURE 30–3.
Primary outcome data from the ventricular restoration arm of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. CABG, coronary artery bypass graft; SVR, surgical ventricular restoration. Reproduced with permission from Jones et al.20
Cardiac Support Devices
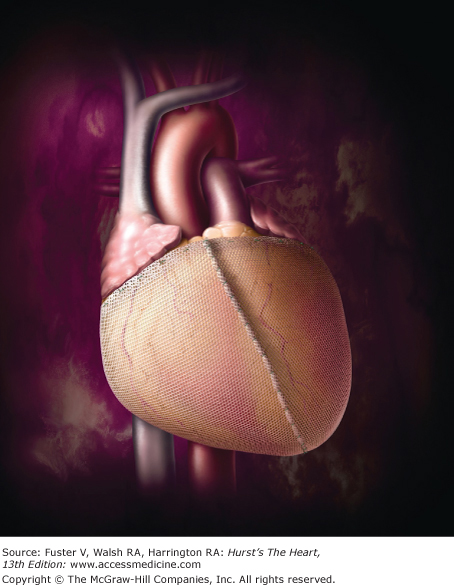
Recent enthusiasm in the concept of ventricular geometric restoration has promoted the development of cardiac support devices (CSDs) that attempt to limit or reverse ventricular remodeling. The CorCap CSD (Acorn Cardiovascular, St. Paul, Minn) provides wall-stress diminution through a circumferential mesh constructed from a multifilamentous yarn that provides high strength and fatigue characteristics to its diastolic support while maintaining flexibility (and thus not causing constriction). It is positioned around the ventricles (similar to a vest), where it is sewn just adjacent to the atrioventricular groove (Fig. 30–4). The device is then adjusted gradually to reduce the circumference of the heart and thereby decrease wall stress. Once this is accomplished, it is secured with several anterior stay sutures; its use does not preclude concomitant coronary or valve surgery.
Promising early data from Europe suggest that the CorCap CSD may provide a durable decrease in ventricular volumes and increase in EF in medium-term follow-up studies.22 Animal studies have suggested that the CorCap CSD may also help to prevent extension of myocardial infarction and resultant heart failure, implying that its efficacy may be of additional benefit to impede the process of ventricular remodeling after a large myocardial infarction.23 In the Acorn trial, 300 heart failure patients were enrolled, including 193 patients who needed a mitral valve procedure and 107 patients who required only a CSD. The patients requiring mitral valve surgery were randomized to mitral surgery alone versus mitral surgery and CSD. The CSD-only patients were randomized between medical therapy and medical therapy plus CSD. In this study, CSD patients required fewer cardiac procedures and experienced reduction in end-systolic and end-diastolic volumes. However, survival and serious adverse event rates were similar between the groups.24
In the subcohort of patients requiring mitral valve procedures, the majority (84%) of patients underwent mitral valve repair with an undersized ring; the remainder underwent mitral valve replacement. Notably, the reduction in mitral regurgitation in both repair and replacement cohorts was durable at the 18-month follow-up, as were findings of improvement in quality of life. Both findings were additionally enhanced when mitral valve surgery was performed in conjunction with application of the CorCap CSD.25 The results of this recent subcohort analysis are compelling for several reasons, including the favorable results of mitral valve surgery in this group of patients with low EFs where perioperative mortality was <2%. However, perhaps most intriguing is the validation of a composite strategy toward the application of surgery for end-stage heart disease. As experience with adjunctive devices and operations grows, it is perhaps logical that the specific cohorts for whom each is most beneficial will be refined. Thus, for the patient with a pure dilated cardiomyopathy and mitral valve regurgitation, the approach of restraint device and mitral valve surgery may be best. If that patient additionally has a ventricular aneurysm and no valvular pathology, ventricular restoration with or without coronary revascularization may be the solution. The complexity of decision making given this wide range of potential solutions for the surgical management of ventricular dysfunction makes scientific evaluation of the various techniques a substantial challenge. Nonetheless, surgeons involved with operations for end-stage heart failure as alternatives to transplantation should avail themselves of the full armamentarium of therapeutic options to obtain optimal outcomes.
Mechanical Circulatory Support
The concept of mechanical circulatory support originated in 1954 with the perfection of the DeBakey roller pump, which became the fundamental component for the early cardiopulmonary bypass machines. Michael DeBakey investigated several assist devices in the animal laboratory before using an external gas-energized synchronized hemispheric pump constructed of Dacron-reinforced silastic to support two patients who failed to wean from cardiopulmonary bypass in 1966.26 The first total artificial heart (TAH), developed by Domingo Liotta in DeBakey’s laboratory, was implanted 3 years later by Denton Cooley in a patient who could not be weaned from cardiopulmonary bypass. This patient was transplanted after approximately 60 hours on the device, but he subsequently died due to pulmonary complications.27 Cooley attempted a second TAH implant in 1981 (using the Akutsu model III artificial heart) that was complicated by device mismatch, requiring the chest cavity to be left open until transplantation, after which the patient died.28 The first permanent implant of a TAH was performed in 1982 at the University of Utah by William DeVries and Lyle Joyce, using the Jarvik-7 device that was a modification of a pump designed by Willem Johan Kolff.29 The patient was turned down by three different transplantation centers and was ultimately supported on this device for 112 days. In 1985, Copeland and colleagues30 reported the first use of this device as a successful bridge to transplantation (BTT). However, subsequent experience with the TAH led to an unacceptably high incidence of thromboembolic events and infections, prompting a shift in focus to the use of LV assist devices (LVADs) to provide mechanical support in patients awaiting heart transplantation. Portner and colleagues31 reported the first use of this strategy, implanting the Novacor left ventricular assist system (with an electromechanically driven dual pusher-plate blood pump) in 20 patients starting in 1984. Early success by Frazier et al32 using a pneumatically driven pulsatile LVAD (HeartMate 1000 IP) contributed to the approval of these devices for BTT in 1994 by the US Food and Drug Administration (FDA). Ultimately, the benefit of using an LVAD to provide long-term support of patients with end-stage heart failure who are not eligible for transplantation or destination therapy was established by Rose et al33 in the landmark Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial. In REMATCH, support with the HeartMate XVE LVAD conferred a 48% survival benefit over 2 years when compared with optimal medical therapy in patients with decompensated end-stage chronic heart failure who were not candidates for cardiac transplantation.33 More recent advances in LVAD technology have permitted the use of smaller second-generation axial flow devices, which pump blood based on an Archimedes screw, rotary pump-type mechanism. A newer third generation of LVADs, which pump blood via a centrifugal flow mechanism, are currently under investigational use.
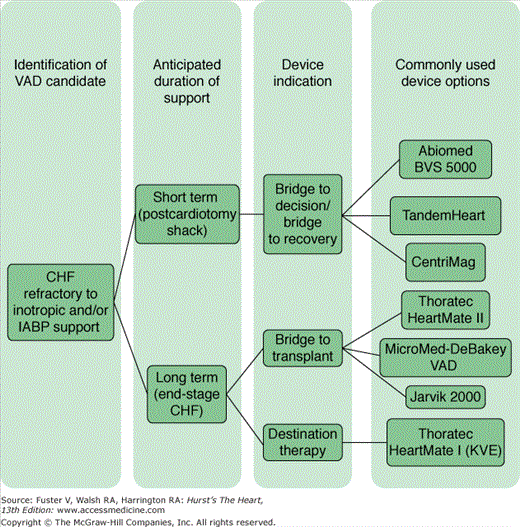
Mechanical circulatory support (MCS) is considered for two primary clinical scenarios: decompensated end-stage chronic heart failure and acute refractory cardiogenic shock. The appropriate indication for device therapy guides the selection of device type. Long-term devices are preferred for patients with chronic heart failure, and short-term devices are used for patients with acute refractory cardiogenic shock. This treatment algorithm is summarized in Fig. 30–5. Other important considerations when choosing a device, apart from expected duration of support, include the need for biventricular support, cost, device-related risks (such as the need for anticoagulation and device failure rates), patient characteristics (especially the size and blood type of the patient), and United Network for Organ Sharing (UNOS) classification rules.34
Patient selection for chronic ventricular assist device (VAD) therapy is evolving. Initial indications were derived from the REMATCH inclusion criteria and include adult patients with chronic end-stage heart failure as defined by a left ventricular EF (LVEF) of ≤25% or symptoms of New York Heart Association (NYHA) class IV for at least 60 days and a peak oxygen consumption of no more than 14 mL/kg/min, as well as patients who have been in NYHA class III or IV for at least 28 days and who have received at least 14 days of support with an intra-aortic balloon pump or have a dependence on intravenous inotropic agents, with two failed weaning attempts despite attempted therapy with ACE inhibitors, diuretics, and digoxin.35 These criteria are summarized in Table 30–1 and represent the most conservative end of the spectrum with regard to patient selection. Many patients who do not exhibit all of these criteria will benefit from LVAD therapy, but these expanded criteria have not been well defined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree