Fig. 25.1
Freedom from additional revascularization among coronary artery disease (CAD) patients in the MASS-II trial who received medical treatment only (MT), coronary artery bypass grafting (CABG), or percutaneous coronary intervention (PCI)
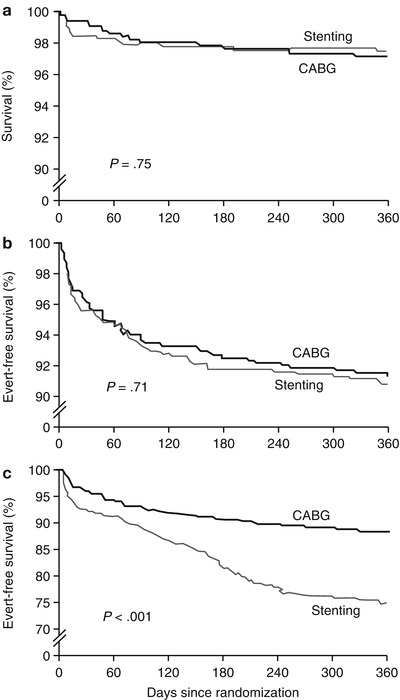
Fig. 25.2
Patients in the ARTS trial who underwent coronary artery bypass grafting (CABG) or catheter-based intervention (CBI) with stenting had similar outcomes when actuarial survival (a) and survival without myocardial infarction or cerebrovascular events (b) were considered. However, when repeat revascularization was combined with these other end points (c), outcomes were better in CABG patients
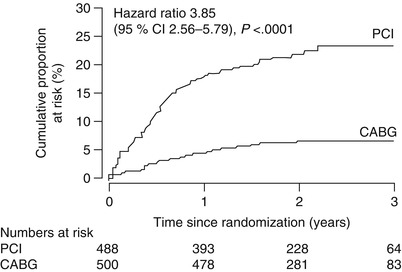
Fig. 25.3
In the SoS trial, risk of repeat revascularization was considerably greater in CAD patients who underwent percutaneous coronary intervention (PCI) than in those who underwent coronary artery bypass grafting (CABG)
Efforts to reduce the rate of revascularization among PCI patients led to the development of drug-eluting stents. These stents are coated with antiproliferative drugs that are gradually released into the stented vessel to prevent restenosis. In recent years, two large-scale randomized trials have produced data about the efficacy of these stents.
The SYNergy between PCI with TAXUS and Cardiac Surgery (SYNTAX) study was an international randomized trial of PCI with a paclitaxel-coated stent in patients with 3-vessel or left-main CAD. In this trial, 1,800 patients (at 85 centers) were randomly assigned to undergo PCI or CABG. The 3-year follow-up data showed that the PCI patients had higher rates of repeat revascularization (19.7 % vs. 10.7 %), as well as myocardial infarction (MI) (7.1 % vs. 3.6 %), than CABG patients [19]. The 5-year follow-up data were similar: PCI patients were more likely than CABG patients to have MI (9.7 % vs. 3.8 %) and repeat revascularization (25.9 % vs. 13.7 %) [20]. The two groups did not differ significantly with regard to all-cause mortality and stroke. A subgroup analysis of the SYNTAX data examined cases of incomplete revascularization, defined as the revascularization of fewer vessels than were originally identified by an interventional cardiologist and a surgeon as needing revascularization. This analysis showed that incomplete revascularization was associated with major adverse cardiac or cerebrovascular events (all-cause death, MI, stroke, or repeat revascularization), but only in patients who underwent PCI, not in CABG patients [21].
The SYNTAX score was developed for use in the SYNTAX trial as a way to quantify the complexity of each patient’s CAD. Stenoses are weighted according to their location and severity as revealed by angiography [22]. The SYNTAX score appears to be useful in predicting adverse outcomes in patients who undergo PCI, because several studies have shown that PCI patients in the top tertile or quartile of SYNTAX scores have the highest rates of mortality, MI, and repeat revascularization at 1- to 5-year follow-up [23–28]. It is less clear whether the SYNTAX score can guide the choice between CABG and PCI; at least two studies have shown that CABG has better outcomes than PCI in patients with high SYNTAX scores [29, 30], but at least one study has found no such difference [23].
A second randomized trial of PCI with drug-eluting stents, the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial, was performed in patients with diabetes who had substantial CAD (>70 % stenosis in at least two coronary arteries, affecting at least two major coronary-artery territories) but no left main coronary stenosis. The 1,900 patients (treated at 140 centers) underwent either CABG or PCI with a drug-eluting stent; most of these stents were coated with sirolimus (51 %) or paclitaxel (43 %). After 5 years of follow-up, the investigators found that the PCI patients had significantly higher rates of all-cause mortality (16.3 % vs. 10.9 %), MI (13.9 % vs. 6.0 %), and stroke (2.4 % vs. 5.2 %) [31]. Thus, the introduction of drug-eluting stents does not seem to have put PCI on par with CABG with regard to long-term outcomes.
Other studies also suggest that CABG is preferable to PCI in certain types of patients. For example, one third of CAD patients who require more than medical therapy alone have chronic total occlusion of one or more vessels [32]. Two trials of CABG and PCI that included patients with totally occluded vessels have shown that PCI does not produce adequate revascularization in these vessels. In the Randomized Intervention Treatment of Angina (RITA) trial [33], only 48 % of attempts to revascularize totally occluded arteries with PCI were considered successful (i.e., reduced stenosis to 50 % or less). In the Coronary Angioplasty versus Bypass Revascularization Investigation (CABRI) trial [34], only 70 % of occluded arteries were successfully revascularized with PCI.
There are other subgroups of CAD patients who may also benefit more from CABG than from PCI. In patients with left main artery disease, this vessel may be particularly vulnerable to restenosis after PCI [35]. Additionally, CAD patients with diabetes mellitus have better 5-year survival after CABG with internal mammary artery (IMA) grafts than after PCI [36].
The data do not universally support the choice of CABG over PCI in patients with severe disease, however. Serruys and colleagues [37] conducted a randomized trial of these two treatments in 1,800 patients with severe CAD (defined as 3-vessel and/or left main disease). Twelve-month follow-up data showed a higher rate of stroke in CABG patients than in PCI patients (2.2 % vs. 0.6 %). Mortality and MI rates were similar between the two groups.
Coronary Artery Bypass Grafting Techniques
The technology used in CABG surgery has evolved substantially during the last three decades. Refinements in operative technique (Figs. 25.4, 25.5, 25.6, 25.7 and 25.8) have continued to improve outcome in a patient population that is increasingly infirm. Meta-analyses of large numbers of CABG studies report 30-day mortality rates in the 1–2 % range [38, 39].
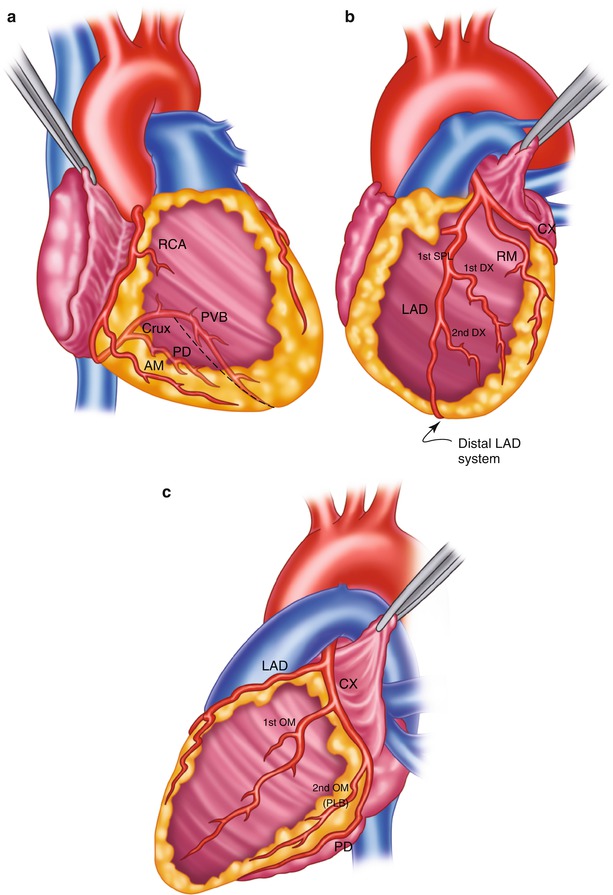
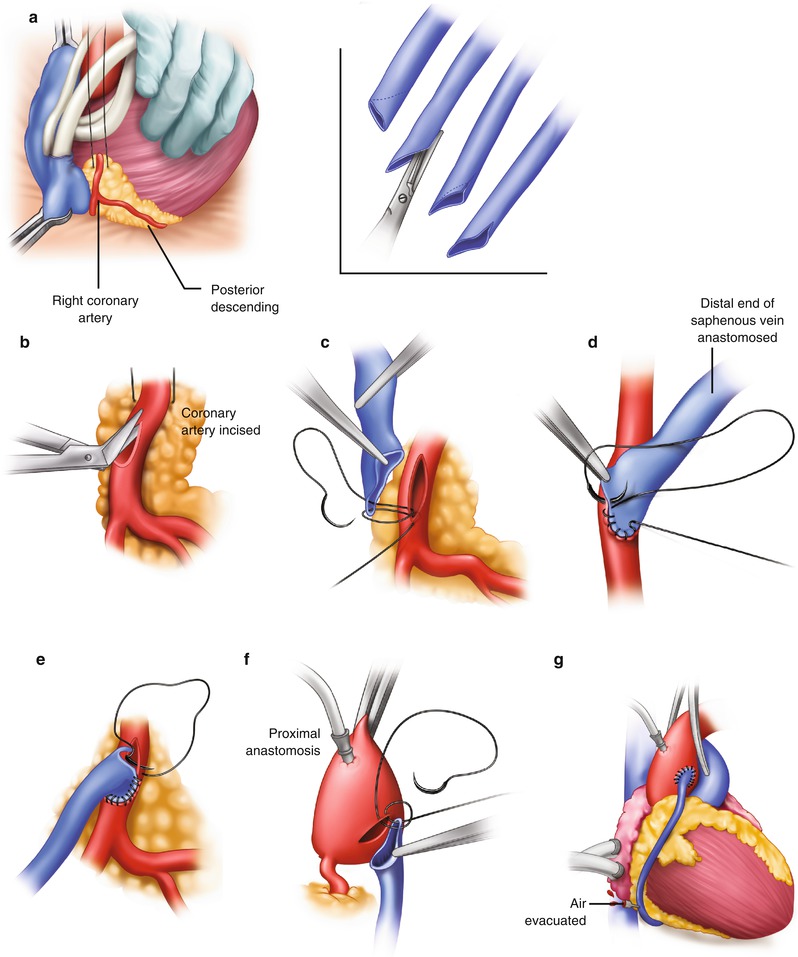
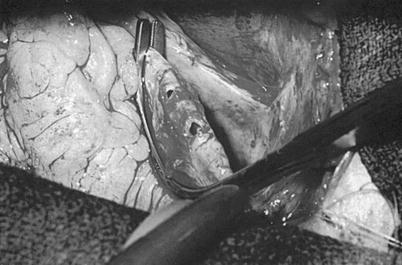
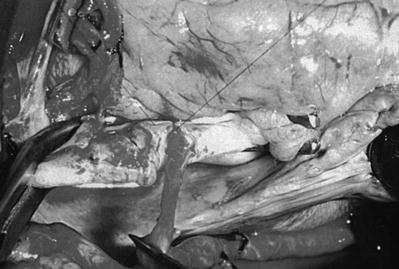
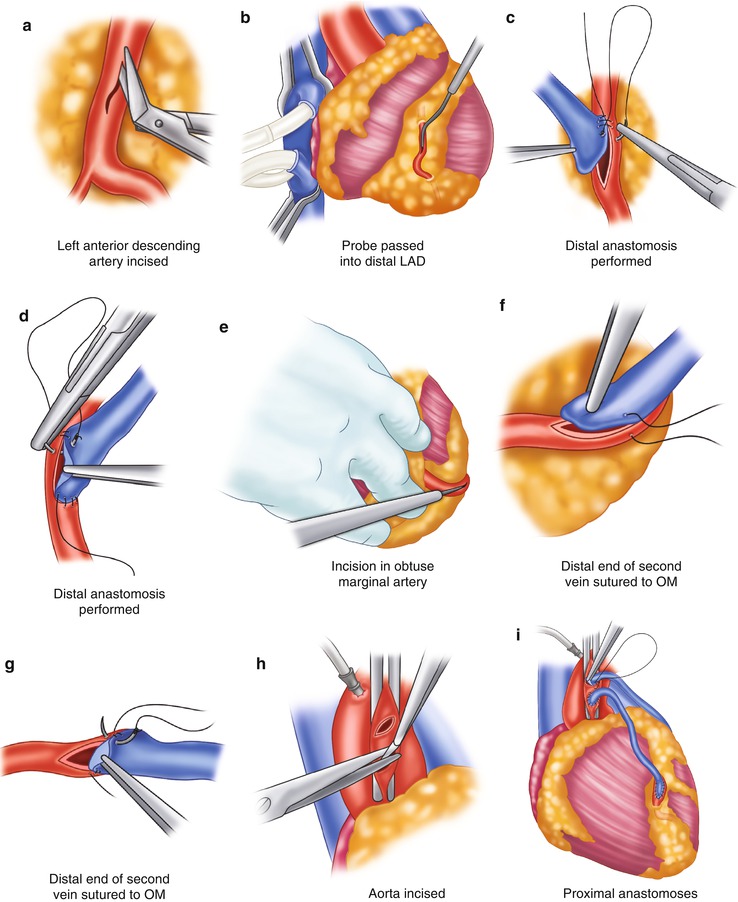

Fig. 25.4
Surgical anatomy of the coronary system. (a) The right coronary system has several branches that can be considered for bypass. In some instances, the acute marginal branch (AM) is bypassed, particularly if it has a large retrograde collateral blood supply to the left system. Most anastomoses are made at the area of the crux. The posterior ventricular branch (PVB), posterior descending branches (PD), or their distal branches may be grafted. The dotted line indicates the area of the interventricular septum as denoted by the right coronary vein. RCA right coronary artery. (b) In the left anterior descending coronary artery (LAD) system, the first septal perforator (1st SPL) is usually just opposite the first diagonal (1st DX) branch or is proximal to it. It descends posteriorly into the interventricular septum and may be an important artery. The 1st DX and second diagonal (2nd DX) branches arise from the LAD. The distal LAD usually supplies the apex of the left ventricle. The ramus medialis branch (RM) arises between the circumflex (CX) and the LAD and may be in the DX or obtuse marginal position. (c) The coronary system shows the first branch as the first obtuse marginal (1st OM) and the second branch as the second obtuse marginal (2nd OM) of the CX system. The main CX trunk is in the atrioventricular groove in close association with the coronary sinus vein

Fig. 25.5
Saphenous vein graft to the right coronary artery. (a) The right coronary artery is isolated by placing a traction suture in the myocardium beneath the right coronary artery. (Inset) The vein is beveled, depending on the length of the anastomosis and the size of the vein. (b) The coronary artery is incised, and the incision is extended with the use of Potts scissors. (c) The right coronary anastomosis is begun at the distal portion of the artery and the toe of the vein graft. (d) The anastomosis is sutured on the right side first, from the artery to the vein. (e) The anastomosis is completed from the left side. The suture is usually tied at or to the right of the apex. (f) The proximal anastomosis is completed with the aid of either complete or partial aortic occlusion. (g) The clamp is removed, and air is evacuated from the vein graft. The vein graft may be clamped with a small bulldog clamp to avoid introducing more air into the coronary system

Fig. 25.6
Incisions are made in the aorta for the proximal anastomoses

Fig. 25.7
Proximal anastomosis of the right coronary artery bypass graft

Fig. 25.8
Saphenous vein graft to the left circumflex system. (a) The left anterior descending coronary artery (LAD) is identified and incised. The incision is enlarged with a Potts scissors. (b) The probe is passed into the distal LAD to ensure patency. (c) The distal anastomosis is created by attaching the heel of the graft to the proximal portion of the artery, from outside the artery to inside the vein. Left-side suturing is completed around the toe of the graft to approximately the midportion of the right side of the artery. (d) The other end of the suture is then sutured from the heel of the graft, passing the vein to the artery. The graft is then tied in the midportion of the artery. (e) To expose the obtuse marginal artery, the heart is retracted to the right by an assistant. The obtuse marginal branch is then incised. (f, g) Anastomosis of the obtuse marginal (OM) branch is begun at the proximal portion of the artery; the heel of the graft is placed to the proximal portion of the artery. Suturing is accomplished on the left side of the graft, from outside the artery to inside the vein. The anastomosis is completed on the right side by suturing from outside the vein to inside the artery. The sutures are usually tied at or near the apex. (h) The aorta is incised. (i) The proximal anastomoses are completed
It is interesting to note that numerous techniques have evolved that seem to produce equally good results. Some centers perform only the distal anastomoses while the heart is arrested and perform the proximal ones with a side-biting clamp and bypass, whereas other centers keep the heart arrested while all grafts are performed. Cardioplegia techniques vary widely among surgeons and institutions in terms of the use of warm blood, cold blood, or crystalloid, the number and timing of doses, and the route by which doses are given (anterograde or retrograde), but there is not widespread agreement on which of these methods is best.
Graft Selection and Patency
One area of bypass surgery in which the evidence does favor particular options is choice of conduit. Saphenous vein harvest has become more sophisticated and less invasive over the past several years, producing venous segments of similar quality to those harvested with open techniques while causing fewer complications [40–42]. However, there is plenty of evidence to suggest that IMA grafts (Fig. 25.9) more effectively promote short- and long-term survival than do saphenous vein grafts, especially when used to graft the LAD (Fig. 25.10) [43–46]. There is now compelling evidence that left IMA (LIMA)-to-LAD grafts have an 88 % patency rate 15 years after surgery (Fig. 25.11) [47]. Grafts constructed from the radial artery may also be associated with better postoperative survival, freedom from adverse events, and long-term graft patency than saphenous vein grafts [44, 48–50], although there is not total agreement on this point [51–53]. Additionally, endoscopic harvesting of radial artery segments appears to produce good results (Fig. 25.12) [54–56]. However, there is some evidence that radial grafts are less effective when used to graft noncritically stenosed vessels or the right coronary artery [47, 50, 57–59].
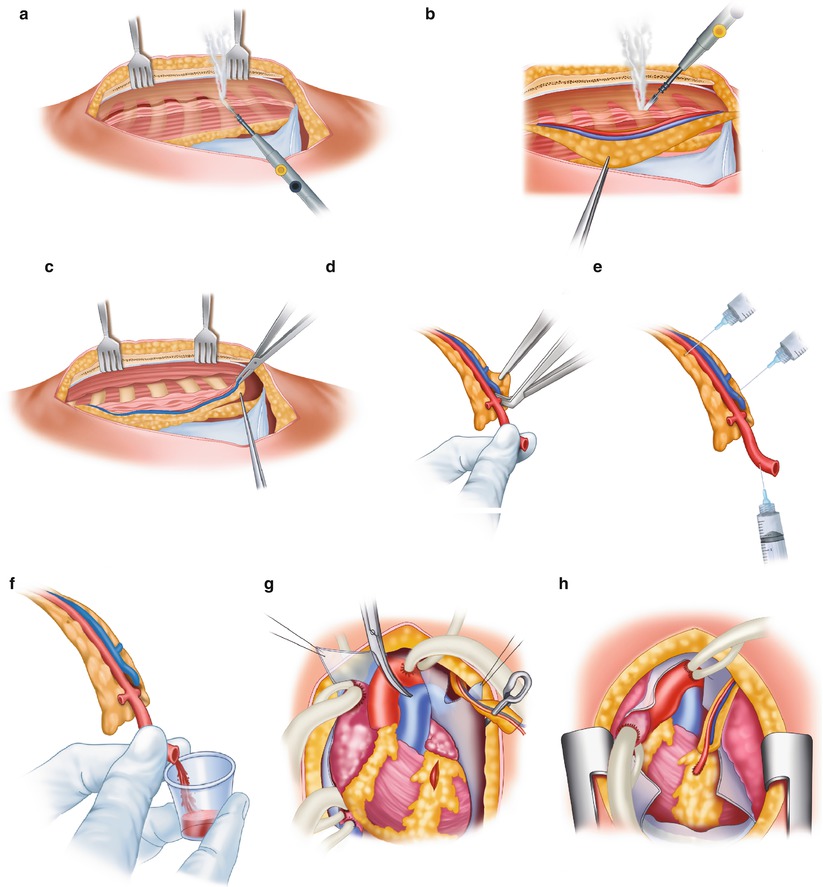
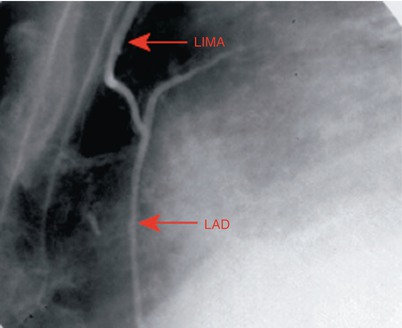
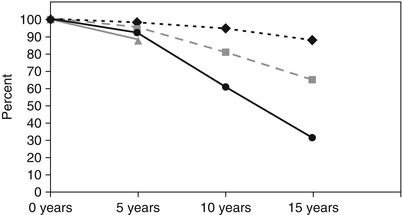
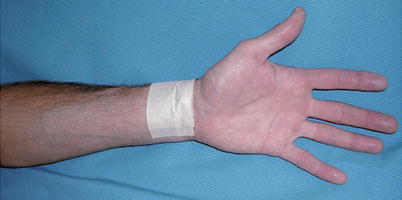

Fig. 25.9
Technique for obtaining the internal mammary artery (IMA) for use as a bypass conduit. (a) The IMA is mobilized from the chest wall with cautery. Parallel incisions are made on each side of the pedicle, which includes the vein, artery, lymphatics, fascia, and muscle. (b) Only the tissue around the IMA should be handled with forceps. The distal intercostal arteries and branches are electrocoagulated. Metal clips or ligatures are placed on the proximal branches. (c) The distal portion of the pedicle around the IMA has been removed, and a metallic clip has been placed on the distal mammary artery. Metallic clips are used to gain hemostasis of the pedicle without trauma to the artery. (d) The pedicle is cleaned of its distal portion, and the artery is exposed. (e) A solution of papaverine (30 mg/100 mL) is injected into the mammary artery with a syringe. Papaverine solution is also applied to the pedicle. (f) After the graft is dilated, flow is assessed. If flow is less than 60–100 mL/min, a more proximal portion with a higher flow rate should be obtained. (g) The LAD is isolated, and the graft is aligned. (h) The anastomosis is completed. Care must be taken to ensure proper alignment of the graft on the myocardium, especially with respect to the pericardium, the pleura, the lung, and the beating heart

Fig. 25.10
Angiogram showing a left internal mammary artery (LIMA) graft to the left anterior descending coronary artery (LAD), which confers a survival advantage over saphenous vein grafts

Fig. 25.11
Data from a large coronary artery bypass grafting study by Tatoulis et al. show the 15-year patency rates of coronary conduits made from the left internal mammary artery (diamonds, n = 1,345), right internal mammary artery (boxes, n = 605), radial artery (triangles, n = 177), and saphenous vein (circles, n = 3,714)

Fig. 25.12
Videoscopic radial artery harvest allows the artery to be removed through a 3-cm incision at the wrist. At many centers, radial artery conduits are thought to be superior to saphenous vein conduits and are used in most cases
Several techniques have been tried to make the patency of vein grafts comparable to that of arterial grafts. In the PREVENT IV trial, a gene transcription factor decoy was used in an effort to minimize intimal hyperplasia in vein grafts, but without success [60]. Subsequently, a laboratory study showed that using nitinol mesh to externally support and constrict a vein graft substantially suppressed intimal hyperplasia [61]. Testing a different approach to preventing vein graft failure, the CASCADE trial attempted to determine whether the use of both aspirin and clopidogrel after multivessel CABG resulted in better saphenous vein graft patency than aspirin alone. Follow-up intravascular ultrasonography found no differences in patency 1 year postoperatively [62]. (Clopidogrel does not appear to improve graft patency after off-pump revascularization procedures, either [63].) What is more, a systematic review by Patel and colleagues [64] found no clear benefit of postoperative clopidogrel use for CABG patients.
Cardiopulmonary Bypass
One of the chief stumbling blocks in producing favorable outcomes after CABG is the necessity of cardiopulmonary bypass (CPB) (Figs. 25.13 and 25.14). Although CPB and cardioplegia remove motion and blood from the surgical field, contact between blood and the artificial materials within the CPB pump can provoke a systematic inflammatory response (SIR) that may cause coagulopathy, third-space fluid retention, and impaired end-organ function, which can lead to significant morbidity in patients with preexisting end-organ dysfunction.
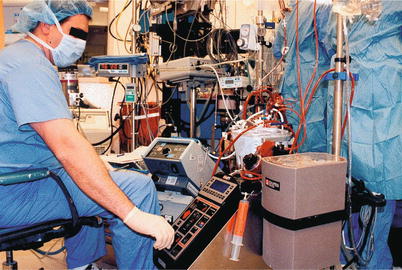
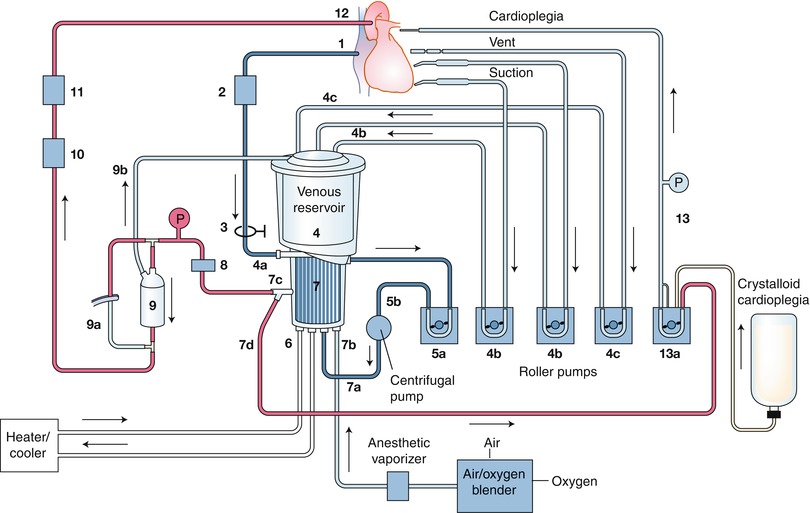

Fig. 25.13
The modern heart-lung machine is highly evolved, with computer-controlled pumps and state-of-the-art materials and design

Fig. 25.14
A schematic of a cardiopulmonary bypass (CPB) circuit. The particular components of CPB circuits vary in several ways according to the individual patient’s circumstances and the surgeon’s preferences, including the type of pump (roller vs. centrifugal) and reservoir (open vs. closed) used, and the routes by which cardioplegia is delivered. The numbers indicate the direction of blood flow
Several technical innovations have been made in an attempt to reduce the negative impact of CPB. These include reducing contact between blood and the CPB mechanism by coating the internal surface with heparin or other polymers [65–67], minimizing hemodilution by shrinking the bypass circuit to reduce the amount of crystalloid prime needed to start CPB [68], and using hemofiltration to protect the heart and end organs [69, 70]. Preliminary evidence suggests that preoperative administration of anti-inflammatory drugs reduces SIR and may improve surgical outcomes [71–74]. Avoiding the use of cardiotomy suction devices (which tend to induce hemolysis) has also been tried with some success [75, 76], and some evidence suggests that using a cell saver instead of a cardiotomy reservoir reduces lipid microemboli [77] and improves the biocompatibility of the bypass circuit [78]. Finally, there is the option to forgo CPB altogether.
Off-Pump Coronary Artery Bypass
Current U.S. estimates (based on industry reports) suggest that 15–25 % of CABG procedures are performed without the use of CPB. The majority of these procedures are performed by a small percentage of heart surgeons who specialize in off-pump coronary artery bypass (OPCAB); the majority of surgeons perform OPCAB in less than 5 % of their patients who require bypass surgery.
Technical Aspects
Keeping the coronary arteries in place during beating-heart bypass necessitates using one of three types of coronary stabilizers: compression-type stabilizers, which use two-pronged forks that grasp the epicardium on either side of the artery to be bypassed; suction-type stabilizers, which use forks whose grasping surfaces have suction ports that improve their grip without the need for greater downward force; and capture-type stabilizers, fenestrated platforms through which silicone elastic tapes are passed, looped under the coronary artery, and pulled tight to hold the epicardium against the underside of the platform (Figs. 25.15, 25.16 and 25.17).
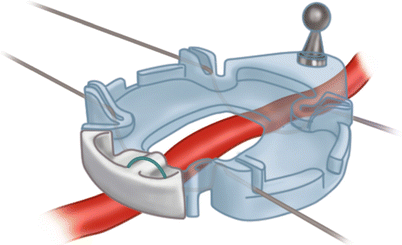
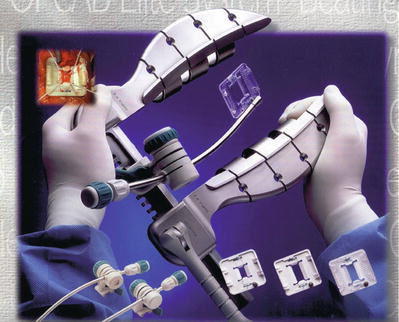
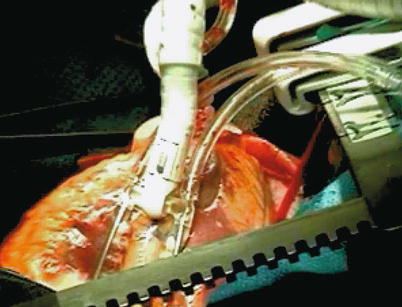

Fig. 25.15
Some stabilizers use a combination of silicone elastic tapes under the artery and a fenestrated platform to provide a motionless, blood-free field

Fig. 25.16
Several self-retaining stabilizers are commercially available. When used correctly, these allow multivessel bypass to be performed safely and reproducibly in the majority of patients. The blood-free, motionless field these devices provide allows anastomotic precision very similar to what is achieved with cardiopulmonary bypass and an arrested heart

Fig. 25.17
The Octopus stabilizer (Medtronic, Minneapolis, MN) uses suction to grip the epicardial surface so that less downward pressure is required, which improves the stability of the coronary artery in some situations
In some patients, the left hemisternum can block the surgeon’s view of the proximal obtuse marginal and posterolateral branches of the circumflex arteries, making it difficult to graft coronary arteries onto the lateral and posterolateral areas of the heart. Attempts to correct this problem by forcing the heart to the right with stabilization devices may compromise both blood flow and stability. However, several devices and techniques have been developed to improve exposure while maintaining hemodynamic stability during OPCAB. Deep pericardial sutures (Fig. 25.18), first described by Lima [79], can be tightened to create a pericardial ridge that supports the base of the lateral left ventricle, allowing the heart to be rotated to the right so that the apex points upward through the sternotomy incision. This provides adequate exposure of the lateral and inferior left ventricle. Apical suction devices, which enable the surgeon to pull the apex of the heart away from the base and pivot the cardiac axis, can also be used for this purpose; animal studies have shown that these devices are as effective as deep pericardial sutures [80, 81] and may cause less hemodynamic interference [80]. When it is difficult to expose the lateral wall, right pleurotomy and right vertical pericardiotomy (Fig. 25.19) may be used in addition to deep pericardial sutures to allow the heart to herniate into the right pleural space while maintaining right ventricular end-diastolic volume (Fig. 25.20). Performing asymmetric right hemisternal elevation in addition to the preceding techniques permits even greater lateral wall exposure, bringing the proximal obtuse marginal and posterolateral branches into the center of the operative field.
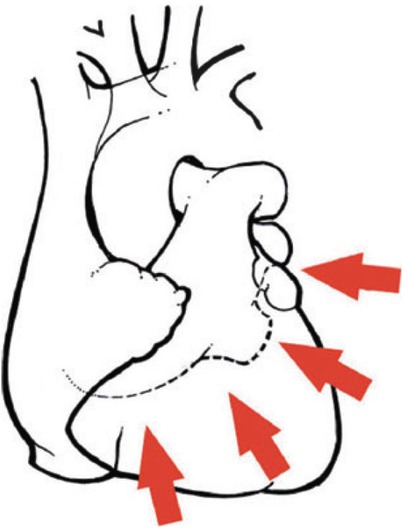
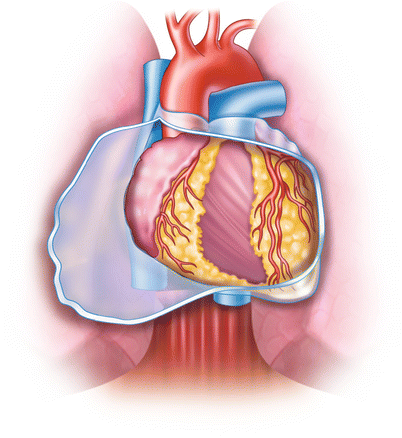

Fig. 25.18
Sutures placed deep in the pericardial sac, when tightened, facilitate rotation of the heart up and to the right. This rotation allows off-pump bypass to be performed to the inferior and lateral aspects of the beating heart without hemodynamic compromise in most patients. Arrows indicate points on the suture line

Fig. 25.19
Vertical pericardiotomy allows the heart to be rotated into the right chest to facilitate exposure of the posterior and lateral aspects during off-pump coronary artery bypass. This technique is particularly useful in patients with large hearts

Fig. 25.20
Elevating the right half of the sternum and widely opening the right pleura and pericardium allows the heart to be rotated into the right chest, producing excellent exposure of the obtuse marginal territory. This patient had little hemodynamic compromise with this maneuver
Maintaining a bloodless surgical field is necessary for constructing the coronary anastomoses during OPCAB. Normally, this is done by using temporary vascular occluders (e.g., clamps, vessel loops, snares, intracoronary shunts) on the coronary arteries. Because these devices can injure the arteries or cause endothelial dysfunction, some investigators have tested the use of a thermosensitive polymer (LeGoo; Pluromed, Woburn, MA), which is liquid at room temperature but solidifies into a gel at body temperature, to prevent blood flow into the surgical field during OPCAB. An initial human trial showed that the polymer could completely block blood flow from the coronary arteries without further intervention in 91 % of attempts [82]. Subsequently, other investigators found that using the polymer more often resulted in satisfactory hemostasis than using vessel loops (88.0 % vs. 60.7 %) and shortened the total anastomotic time (12.8 vs. 15.1 min). Thirty-day adverse event rates did not differ significantly between the two groups [83]. However, the polymer is not necessarily a perfect alternative to occlusive devices; results of an ex vivo study of the polymer’s effect on the IMA indicate that it may cause endothelial injury and smooth muscle dysfunction [84].
Results of Off-Pump Coronary Artery Bypass
As stated in the 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery [85], there is not yet complete agreement about the relative safety and efficacy of OPCAB and conventional CABG with CPB. The difficulty in comparing the two approaches stems in part from the low incidences of mortality and significant morbidity associated with both techniques [86–97]. However, in the past decade, several large clinical trials, meta-analyses, and registry studies have been performed that had sufficient sample sizes to address this problem. A 2005 meta-analysis of 37 randomized, controlled trials of OPCAB suggested that it is associated with significantly decreased atrial fibrillation, transfusion and inotrope requirements, respiratory infections, ventilation time, intensive care unit stay, hospital stay, and overall in-hospital and (1-year) post-discharge costs compared with standard CABG [39]. Likewise, a randomized controlled trial (CORONARY) performed in 4,752 patients at 19 centers associated OPCAB with less blood transfusion, lower frequencies of reoperation for bleeding, and lower 30-day rates of acute kidney injury and respiratory complications than CABG [98]. These findings may be explained by the results of several small randomized trials, which suggest that OPCAB is associated with lower postoperative CK-MB [97, 99, 100] and troponin levels [97, 101] and less activation of complement factors C5a and TCC [102] than is standard CABG. Additionally, these studies suggest that OPCAB patients may have less blood loss during surgery [100] and a higher postoperative hematocrit [97].
Other studies of the short-term outcomes of CABG and OPCAB have produced less definitive results. Several studies have found in-hospital or 30-day mortality to be lower with OPCAB than with CABG [103–106], but a similar number of studies found no difference between the two procedures [98, 107–110]. With regard to short-term stroke risk, a meta-analysis of 59 randomized trials (n = 8,961) associated OPCAB with a 30 % reduction in 30-day stroke risk [107]. This is consistent with the results of two large, propensity-matched registry studies (CREDO-Kyoto [111] [n = 6,323] and a 2008 study by Puskas et al. [104] [n = 12,812]) and a large retrospective study (n = 13,108) [105], all of which showed lower short-term stroke rates in OPCAB patients than in CABG patients. In contrast, the ROOBY trial [109], the CORONARY trial [98], and a very large (n = 63,047) Veterans Administration registry study [108] found no differences in short-term stroke rates.
With regard to long-term outcomes, the evidence is similarly mixed. One study found that 10-year survival was somewhat poorer in OPCAB patients than in propensity-matched CABG patients [112]. However, Puskas et al.’s registry study [104] and the SMART trial [104] found no differences in long-term survival. This is despite the fact that several studies have associated CABG with better short- and long-term graft patency than OPCAB [109, 110, 113], although this finding is not universal [114].
Among CABG and OPCAB patients surveyed an average of 10.8 months after surgery, OPCAB patients reported having better physical functioning and emotional well-being [115]. However, other studies found that choice of procedure made no difference in terms of quality of life at 18 months [116] or in functional class or treadmill performance at 1 year [110].
Two retrospective studies have identified gender differences in CABG and OPCAB outcomes. In both studies, OPCAB was associated with lower 30-day mortality than CABG, but only in women [103, 104]. One of these studies also found that OPCAB enhanced 1-year survival in women [103]; the other study found that women had poorer 10-year survival than men regardless of which procedure they underwent [104].
A few studies have compared the results of CABG or OPCAB with those of PCI. One such study found that that OPCAB and percutaneous angioplasty with sirolimus-coated stents were associated with similar early and late mortality rates, but that the OPCAB patients had a lower rate of reoperation and return of angina [117]. Likewise, a registry study that found no differences between CABG and OPCAB in terms of outcomes showed that both procedures produced lower 18-month revascularization rates than PCI [116]. In contrast, a single-center, retrospective study found that the 2-year rates of survival and freedom from major adverse cardiac events were both higher in patients who underwent bypass procedures (83 % of which were OPCAB) than in patients who underwent PCI [118].
Early OPCAB procedures were limited in terms of the adequacy of revascularization and the quality and reproducibility of the distal anastomosis, leading surgeons to perform slightly fewer grafts in OPCAB patients than in conventional CABG patients [92, 93]. However, subsequent refinement of devices and techniques for lateral and inferior wall exposure (see above) has enabled surgeons experienced with OPCAB to perform grafts to all aspects of the heart, and recent randomized trials show that the average number of grafts constructed is at most only slightly smaller in OPCAB procedures than in CABG operations (the largest difference being 3.0 for OPCAB vs. 3.2 for CABG) [98, 109, 114].
Several retrospective nonrandomized series have shown that OPCAB may be associated with decreased operative risk in some high-risk groups [86, 119–121], including elderly patients, patients with reduced ejection fraction, and patients with renal failure [87, 122–127]. In addition, OPCAB may be well suited for patients with left main disease [128]. Furthermore, several reports have documented that OPCAB is associated with decreased risk in repeat CABG patients [129–131]. This is because reoperative OPCAB often can be performed without dissecting out the ascending aorta or manipulating diseased grafts. It is less clear whether OPCAB is better than standard CABG for patients with left ventricular dysfunction [132, 133], although such patients may benefit from OPCAB with temporary support from a ventricular assist device [134].
In dealing with heavily diseased but patent vein grafts, many surgeons reject OPCAB because of the risk of graft embolization while the heart is being repositioned. However, in patients with patent LIMA-to-LAD grafts, a tailored left thoracotomy approach for reoperative grafting of the lateral wall allows the construction of a graft from the descending thoracic aorta to lateral wall branches and avoids the potential complications of LIMA-graft mobilization [135, 136]. Similarly, lower sternotomy and upper abdominal incisions have been described for constructing grafts to the inferior wall with the right gastroepiploic artery [137, 138]. Long-term patency data for these types of grafts are not yet available.
Off-pump bypass is ideal for patients with significant atheroma or calcification of the ascending aorta. In these cases, free grafts are attached either to the side of the in situ LIMA, as first described by Tector and colleagues [139], or to the innominate, subclavian, or axillary artery if it is disease-free [140]. Alternatively, free grafts can be attached to a disease-free area of the aorta with an automated anastomotic device [141], or with a clampless anastomotic facilitator that obviates the need for a partial-occluding clamp. Recent series suggest that using these clampless approaches to OPCAB results in low perioperative stroke and mortality rates [141, 142], perhaps lower than those associated with conventional CABG [143, 144]. Palpation and epiaortic echocardiography, when used together, can frequently identify an appropriate dime-sized area of relatively normal aorta. Using OPCAB techniques enables relatively easy revascularization of these high-risk patients and avoids certain hazards associated with cannulation and cross-clamping of the diseased aorta.
Neurocognitive Functioning After Off-Pump Coronary Artery Bypass
The incidence of new onset of subtle neurocognitive dysfunction (NCD) after CABG has been reported to be between 5 and 60 %, depending on the sensitivity of the tests used. Specific deficits include short-term memory loss, impairment in performing simple calculations, and personality and mood disturbances [145–149]. Many factors related to CPB have been implicated, including SIR, alterations in cerebral blood flow, and microembolization caused by the CPB circuit or by cannulation and cross-clamping [147, 149, 150].
A few studies have associated OPCAB with a lower incidence of NCD than conventional CABG with CPB [150–152]. However, other studies have shown no significant difference [109, 110, 153–158]. The difference may be partly attributable to the time frame examined; a meta-analysis of 13 studies by Sun et al. [159] showed that CABG patients had more NCD than OPCAB patients did during the first 3 months after surgery, but this difference was no longer apparent when the patients were tested 6 months and 1 year postoperatively. Although several groups have reported that OPCAB is associated with significantly less SIR than is CABG [87, 160, 161], the magnitude of SIR has not been shown to correlate with the severity of NCD in individual patients [162]. The failure of OPCAB to reduce postoperative NCD has been largely attributed to the liberation of microemboli during application and removal of the partial-occlusion aortic clamp. Although OPCAB eliminates the need for a perfusion catheter for CPB or a cross-clamp for cardiac arrest, using the side-biting partial occlusion clamp, which is routinely done at many centers during the construction of proximal anastomoses, may be equally traumatic [163]. However, at least one small, randomized, prospective study has associated OPCAB with reduced microembolization—but not a reduced incidence of NCD—compared with standard CABG [164]. In several series, completely eliminating aortic manipulation by performing OPCAB with the exclusive use of in situ and composite grafts produced significantly lower rates of stroke than conventional CABG with CPB or OPCAB performed with a side-biting clamp [164–167]. Additional series in which aortic manipulation was minimized by using an automated anastomotic device (Cardica Inc., Redwood City, CA) to perform the proximal anastomosis or in which the Heartstring proximal seal system (Maquet Cardiovascular, Wayne, NJ) was used to avoid application of the partial occlusion clamp have shown similar results [141–144].
Reducing the Invasiveness of Coronary Artery Bypass
Although median sternotomy is a very well-tolerated incision, it does restrict patients from heavy lifting for 8 weeks and is associated with some degree of perioperative discomfort. In addition, postoperative wound complications, although infrequent, can be serious and may necessitate surgical revision or even sternal resection and plastic surgical reconstruction with tissue-transfer flaps. Patient perception of a 20-cm incision and a “cracked” chest are also important considerations. Furthermore, some data suggest that the magnitude of musculoskeletal trauma is proportional to systemic inflammation [168, 169]. As such, many attempts to refine coronary bypass procedures have focused on finding ways to decrease the size of the incision and the degree of morbidity associated with it.
Minimally Invasive Direct Coronary Artery Bypass
First introduced by Benetti and colleagues in 1995 [170], the minimally invasive direct coronary artery bypass (MIDCAB) procedure involves performing a 7-cm anterior lateral thoracotomy, usually in the 5th intercostal space. By this route, single-vessel off-pump bypass can be performed, using the LIMA to bypass the LAD. Several early reports showed that this technique substantially shortened hospital length of stay relative to standard CABG, and it also reduced transfusion requirements [135, 171–173]. One study also associated MIDCAB with a reduced incidence of atrial fibrillation [174], but two other studies did not support this finding [175, 176]. Results of small randomized trials suggest that, in patients with stenosis of the proximal LAD, MIDCAB is associated with a longer in-hospital recovery period [177] and higher costs than is direct stenting [178], but that MIDCAB produces similar or better short- and long-term outcomes [179, 180]. Nonetheless, in the United States, only a few centers regularly perform MIDCAB today.
Early in the MIDCAB experience, many U.S. centers mobilized the LIMA by using direct visualization through the thoracotomy (Figs. 25.21 and 25.22). This method was often technically demanding, and the forceful retraction of the chest wall necessary to expose the LIMA could cause substantial early postoperative pain. Furthermore, LIMA grafts produced by this technique may be too short. Nevertheless, U.S. centers that perform MIDCAB have frequently reported excellent results [181–185].
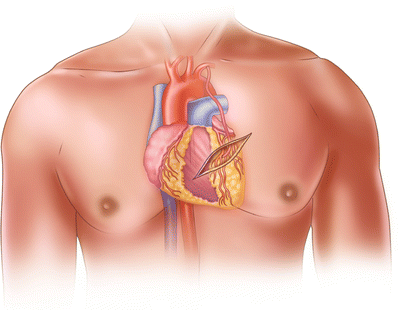

Fig. 25.21
When minimally invasive direct coronary artery bypass was first being used, surgeons harvested the left internal mammary artery through a small anterior thoracotomy by aggressively retracting the anterior chest wall. This technique caused a great deal of postoperative discomfort for the patient and was sometimes technically challenging for the surgeon

Fig. 25.22
Diagram of the minimally invasive direct coronary artery bypass operation, showing the typical size and location of the incision and its position relative to the IMA-to-LAD graft
More recently, there has been resurgent interest in videoscopic LIMA mobilization (Figs. 25.23, 25.24 and 25.25). With this technique, the left lung is collapsed while right-lung ventilation is maintained, the left pleural space is insufflated with CO2, and the mammary artery is harvested from the back of the chest wall with 5-mm videoscopic instruments. This technique avoids the aggressive chest wall retraction required for direct mammary artery harvest and allows the entire length of the artery to be safely mobilized. Although this technique is associated with a significant learning curve, its success has been well documented [186–190].
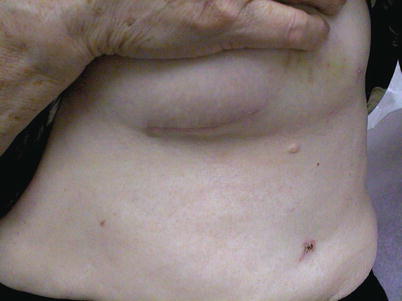
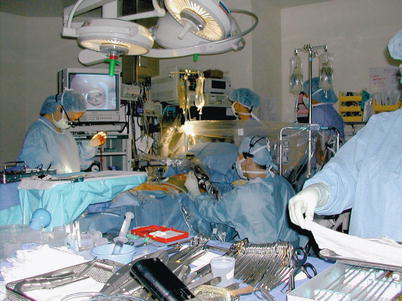
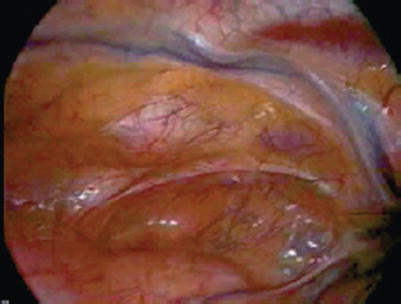

Fig. 25.23
When videoscopic internal mammary artery harvest is combined with off-pump bypass during anterior thoracotomy, the patient experiences only modest postoperative discomfort and a favorable cosmetic result

Fig. 25.24
When a conventional thoracoscope is coupled to a video camera and thoracoscopic instrumentation, the internal mammary artery can be harvested without aggressive chest wall retraction. Single right-lung ventilation and insufflation of the left chest with low pressure CO2 facilitates this technique

Fig. 25.25
Thoracoscopic view of the left internal mammary artery from inside the left chest. Generally, any segment of the left internal mammary artery can be harvested using this technique, but there is a significant learning curve
Hybrid Minimally Invasive Direct Coronary Artery Bypass/Endovascular Procedures
For patients with multivessel CAD, combining a LIMA-to-LAD bypass (i.e., MIDCAB) with endovascular intervention in the circumflex and right coronary arteries may be a good alternative to standard CABG. A multicenter series [191] and a small, single-center series [192] have shown that videoscopic mobilization of the LIMA and opening of the pericardium, using a voice-operated robotic arm to manipulate the scope, enables the surgeon to place the chest wall incision more accurately. This permits construction of the LIMA-to-LAD anastomosis without a rib spreader; instead, only soft tissue retractors and a specially-designed, bed-mounted stabilizer are used to expose the anastomotic site through the natural intercostal space [188]. These patients are also taken to the catheterization lab, where stents are placed in the circumflex and right coronary arteries. This is typically done several days or even weeks before or after the surgical portion of the procedure [193, 194], although both stages can be performed on the same day [195]. The hybrid MIDCAB approach significantly reduces postoperative pain [196], and preliminary patency and outcome data seem promising [194], but comparative studies are needed to justify the expense associated with this approach before it can be widely adopted.
Robot-Assisted Endoscopic Coronary Artery Bypass Grafting
Several reports have documented totally videoscopic LIMA-to-LAD procedures in which surgical robots were used to mobilize the LIMA and perform the sutured anastomosis, both on-pump [197–199] and off-pump (Figs. 25.26 and 25.27) [197, 200, 201]. These robots use master-slave servomotors and microprocessor control to precisely emulate and miniaturize the surgeon’s movements, enabling the surgeon to manipulate videoscopic instruments with multiple degrees of freedom (Fig. 25.28). A high-resolution, 3-dimensional display provides stereopsis with a high degree of magnification (Fig. 25.29). Tremor filtering and motion scaling can be adjusted to the surgeon’s preferences, enabling tremendous precision, which compensates somewhat for the lack of tactile feedback (although tactile feedback systems are under development [202]).
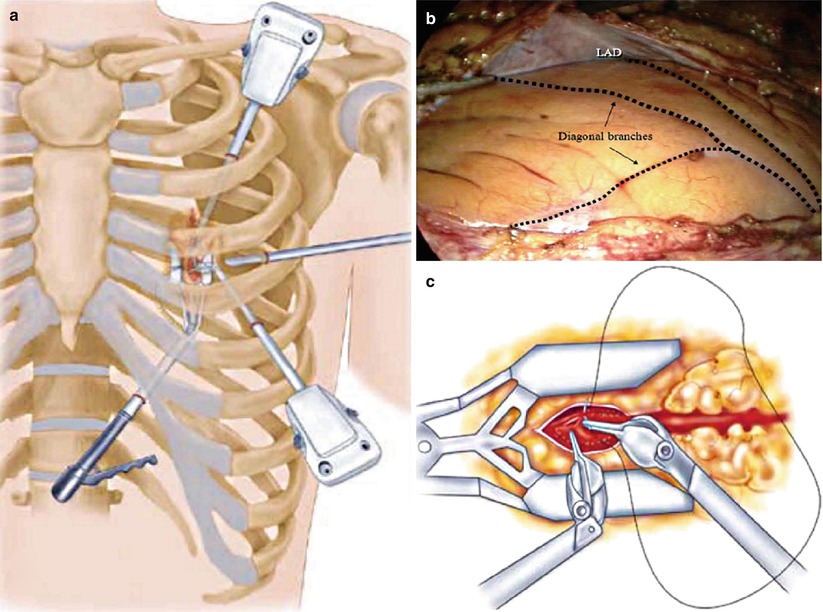
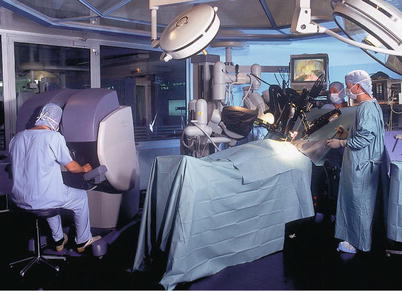
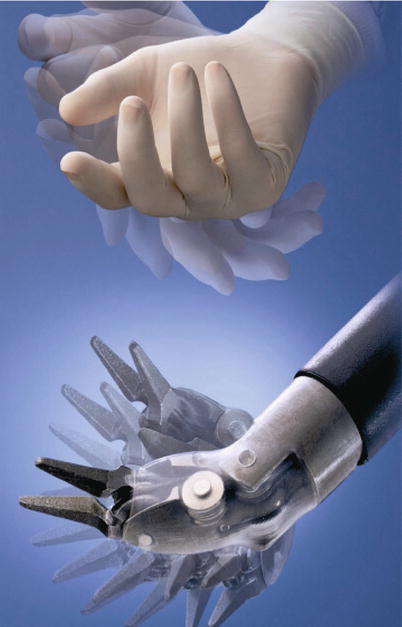

Fig. 25.26
Robotic coronary artery bypass grafting. (a) A 30° scope, two instrument ports, and a subxiphoid endostabilizer are inserted into the left chest via four small incisions, (b) permitting visualization and manipulation of the left internal mammary artery (LIMA) and coronary vessels, including the left anterior descending coronary artery (LAD). (c) The arteriotomy, mobilization of the LIMA, and anastomosis are completed endoscopically

Fig. 25.27
The Intuitive robot (Intuitive Surgical, Inc., Sunnyvale, CA) has a remote console that provides the surgeon with excellent three-dimensional stereopsis and effectors for manipulating the robotic instruments in an intuitive environment. The bedside robotic component is connected to the console by cables

Fig. 25.28




The Intuitive robot uses sophisticated microprocessor control and master-slave servos to emulate the motion of a human hand. This ingenious mechanism provides seven degrees of freedom, enabling surgeons to learn quickly to manipulate the effectors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


