Surgical Treatment of Carotid and Peripheral Vascular Disease: Introduction
Over the past 50 years, the discipline of vascular surgery has witnessed a rapid proliferation and remarkable progress in technique, technology, and research. Advances in anesthesia and perioperative care have further improved mortality and morbidity. In the past 2 decades, endovascular therapy has become a fundamental part of the vascular specialist’s practice, and evidence-based studies have provided improved data to formulate standards of care. The end result has been improvement in the clinical care of patients. This chapter limits itself to four topics in vascular surgery: (1) carotid revascularization; (2) upper extremity revascularization; (3) management of aortoiliac disease and lower extremity revascularization; and (4) upper and lower extremity venous thrombosis.
Carotid Revascularization
Although the percentage of deaths resulting from stroke continues to decrease over time, stroke remains the third leading cause of death in the United States, with a mortality of approximately 45 deaths among a population of 100,000 people. The incidence is increasing, with nearly 800,000 new cases per year in the United States.1 Substantial morbidity also results from stroke, as approximately 31% of all stroke patients require outpatient rehabilitation; furthermore, >18% of patients are unable to return to work.2 Overall, the national 2009 direct and indirect estimate of cost of stroke is $68.9 billion, most of which results from a loss of earnings due to the disability of stroke.1
Approximately 15% to 20% of strokes originate from carotid atherosclerotic plaques, emboli, or thombi.3 There are several preoperative imaging modalities available to evaluate the carotid circulation. Arteriography remains the gold standard of preoperative imaging for carotid artery disease; however, due to the intrinsic stroke risk (0.7%-1.2%)4,5 and cost, arteriography is being used less frequently.3 Instead, vascular surgeons rely on duplex ultrasound alone6 or in conjunction with computed tomography angiography (CTA) and magnetic resonance angiography (MRA).3 Noninvasive studies fail to elucidate all carotid stenoses accurately; therefore, we use carotid angiography when there is:
- Uncertainty about the accuracy or reliability of the vascular ultrasound results
- Uncertainty about the possibility of complete occlusion of the carotid artery in a patient with ongoing localizing symptoms
- Concern about proximal or intrathoracic disease
- A patient with technically difficult studies caused by variant arterial anatomy
- A patient with symptoms and an indeterminate noninvasive study
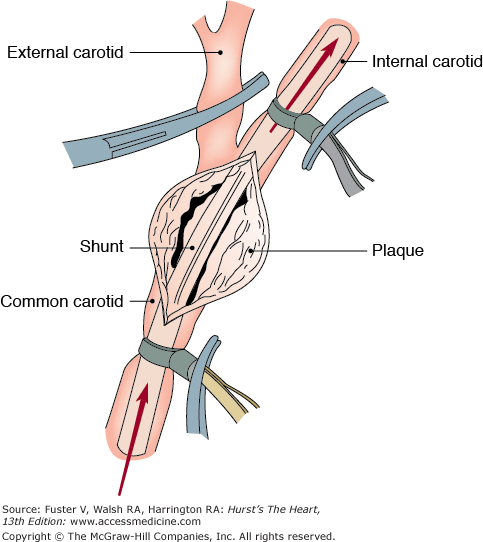
Carotid endarterectomy (CEA) remains the most frequently performed procedure to prevent stroke, with approximately 99,000 operations performed in 2006.1 The operation can be performed under local anesthesia, cervical block, or general anesthesia. Although general anesthesia has the advantage of improved airway control and patient comfort, it does require the use of routine or selective shunting (Fig. 110–1). Selective shunting may involve the use of intraoperative electroencephalography,7 measurement of internal carotid artery stump pressures, or transcranial Doppler ultrasound to assess the need for a shunt.
CEA is performed through a vertical incision along the anterior border of the sternocleidomastoid muscle. The endarterectomy is carried out along a dissection plane in the media of the artery, with the key to the procedure being the attainment of a smooth tapering end point into the internal carotid artery. It is also our practice to place a Dacron or bovine pericardial patch in the majority of our patients because the overall incidence of recurrent carotid stenosis has been shown to be one-third less frequent when compared with primary repair.8,9 Addition of a patch adds only a few minutes to the operation with no significant change in the perioperative morbidity or mortality rate. It is important to note that autogenous patches are prone to pseudoaneurysm formation, which is avoided with the use of synthetic patches. However, because the patch is a foreign body, we adhere to American Heart Association guidelines regarding antibiotic prophylaxis prior to dental procedures or other invasive exams, such as colonoscopy.10
Postoperatively, the patients must be monitored carefully, with frequent neurologic assessment. Hemodynamic monitoring should focus on maintaining the patient’s blood pressure at its preoperative level. Patients should also be watched for the development of a neck hematoma. It is our practice to routinely place a drain in the operative site to minimize hematoma formation and, potentially, subsequent airway compromise. The drain is usually removed the following day.
There have been multiple randomized, controlled studies favoring CEA over medical therapy in both asymptomatic and symptomatic patients (Table 110–1). The following trials also highlight several subgroups that deserve special attention. Women derive less of a benefit from CEA among asymptomatic patients.12,13 Interestingly, women with 50% to 69% symptomatic stenoses also did not show a clear benefit from CEA.17 CEA also provides a greater benefit in patients with hemispheric strokes or transient ischemic attacks compared with patients with retinal ischemic events.17 Patients with contralateral carotid occlusion derive less of a benefit from CEA, particularly patients who are asymptomatic at presentation.11 Furthermore, the data regarding technical outcomes in the surgical arms are derived from centers of excellence; therefore, replication of these outcomes requires referral to vascular surgeons with similar success rates.17 The trials also show that the patients deriving the greatest benefit from CEA are those who are able to survive for at least 2 years. One must always weigh the risks of surgery against the potential benefits. Finally, the medical arms of these trials are outdated, because best medical therapy has been evolving over the past decade. In particular, statin agents, thiazolidinediones, and antiplatelet therapies are being used more frequently. Future studies will be needed to clarify the role of CEA versus modern medical management. With these facts in mind, our current decision-making process regarding carotid revascularization is outlined in Table 110–2.
Stroke Rate | ||||
|---|---|---|---|---|
| Study and Year | No. of Patients | Medical | CEA | |
| Asymptomatic | MACE,11 1992 | 158 | 3.0% | 0.0% |
| Veterans Affairs,4 1993 | 444 | 9.4% | 4.7% | |
| ACAS,12 1995 | 1662 | 11.0% | 5.1% | |
| ACST,13 2004 | 3120 | 11.0% | 3.8% | |
| Symptomatic | ECST (Midterm),14 1991 | 2518 | 16.8% | 2.8% |
| NASCET (70%-99%),15 1991 | 659 | 13.1% | 2.5% | |
| ECST (Final),16 1998 | 3024 | 26.5% | 14.9% | |
| NASCET (50%-69%),17 1998 | 2226 | 22.2% | 15.7% | |
| Category of Patient | Treatment |
|---|---|
| Patients with symptomatic carotid stenosis | |
| >80% stenosis of internal carotid artery | CEA/CAS indicated |
| 50%-79% stenosis of carotid artery but with vascular laboratory data suggesting closer to 79% | CEA/CAS probably indicated; assess risk factors |
| 50%-79% stenosis of carotid artery but with vascular laboratory data suggesting closer to 50% | CEA/CAS may be indicated; assess risk factors |
| <50% stenosis of carotid artery | Trial of medical therapy |
| Patients with asymptomatic carotid stenosis | |
| >80% stenosis of carotid artery | CEA/CAS indicated |
| 50%-79% stenosis of carotid artery but with vascular laboratory data suggesting closer to 79% | CEA/CAS may be indicated; assess risk factors |
| 50%-79% stenosis of carotid artery but with vascular laboratory data suggesting closer to 50% | Revascularization not indicated |
| <50% stenosis of carotid artery | Revascularization not indicated |
The data over the past decade regarding carotid artery stenting (CAS) reflect changes in technology. Initial attempts at carotid artery angioplasty and stenting were performed without cerebral protection, with relatively inexperienced interventionalists and primitive equipment. These trials cited unacceptable stroke rates and are presently of historical interest only.18 The current registry and randomized, controlled data all use a cerebral protection device to prevent periprocedural emboli from escaping into the cerebral circulation and require practitioners to demonstrate proficiency with the most recent techniques and equipment. None of these trials shows superiority of CAS, with several showing superiority of CEA (Table 110–3). These trials have been widely criticized, with detractors citing flaws in patient enrollment, study design, lack of long-term follow-up, and definition of end points. After many years of data accumulation and analysis, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) trial results were finally published in July of 2010. While there was a higher risk of stroke with stenting and a higher risk of myocardial infarction with carotid endarterectomy, “the risk of the composite primary outcome of stroke, myocardial infarction, or death did not differ significantly” in patients undergoing either carotid artery stenting or endarterectomy.25 Moreover, future studies will be required as newer modalities and techniques arise to improve the safety of CAS. Presently, we reserve carotid stenting for a select minority of patients who meet criteria for carotid revascularization and who are high risk for CEA. One review suggested the following criteria as guidelines for patient selection26:
- Lesion at C2 or higher, or below the clavicle
- Prior radical neck dissection or neck irradiation
- Contralateral carotid occlusion
- Recurrent carotid stenosis
- Contralateral laryngeal nerve palsy
- Presence of a tracheostomy
- Age ≥80 years
- Class III/IV heart failure or angina pectoris
- Left main or two-vessel or greater coronary artery disease
- Open heart surgery within 6 weeks
- Myocardial infarction within 30 days
- Left ventricular ejection fraction ⩽30%
- Severe chronic lung or renal disease (dialysis dependent)
| 30-Day Stroke/Death Rate | ||||
|---|---|---|---|---|
| Study and Year | No. of Patients | CAS | CEA | |
| Randomized | SAPPHIRE,19 2004 | 334 | 4.8% | 5.4% |
| SPACE,20 2006 | 1200 | 7.7% | 6.5% | |
| EVA-3S,21 2006 | 527 | 9.6% | 3.9% | |
| Prospective series | ARCHeR,22 2006 | 581 | 6.9% | NA |
| Registry | CREATE,23 2006 | 419 | 5.2% | NA |
| CAPTURE,24 2007 | 3500 | 5.7% | NA | |
A more recent review from Italy, however, stated that most patients with prior neck surgery can still undergo CEA with little risk.27 Like most issues in vascular surgery, a thoughtful assessment of risk factors and careful patient selection remain the key to good outcomes. For all patients, clopidogrel therapy is recommended before carotid stent placement and for at least 30 days postoperatively. (For further reading regarding minimally invasive management of carotid artery disease, see Chap. 108.)
Upper and Lower Extremity Revascularization
Symptomatic ischemia afflicts far fewer patients in the upper extremities than in the lower extremities. Excluding cases performed for thoracic outlet syndrome, <5% of all vascular surgery procedures are performed for upper extremity ischemia.28 Although atherosclerosis remains a common cause of upper extremity ischemia, other etiologies, such as collagen vascular diseases, Buerger disease, calciphylaxis, and dialysis access steal, are also highly prevalent. Moreover, complications due to chronic trauma are more prevalent in the upper extremities and must also be considered in the differential diagnosis.28,29
Effort fatigue is a frequent complaint resulting in the need for revascularization, because symptoms of upper arm fatigue are often more functionally disabling than claudication in the lower extremities.28 Other symptoms of upper extremity ischemia requiring revascularization include vertebrobasilar steal, rest pain, and gangrene. A history of prior trauma, occupation, and prior catheterizations of the brachial artery should be elicited. Important physical findings include the pulse examination of both upper extremities and ulceration or gangrene of the digits. Findings on history and physical examination are often subtle due to rich collateral networks surrounding the shoulder and elbow. Therefore, arteriography is required in most cases to confirm the diagnosis and to appropriately plan operative interventions. Other noninvasive tests, such as segmental blood pressure measurements, ultrasound, and computed tomography, play large roles in the diagnostic workup of upper extremity ischemia.
Revascularization depends on the location of the culprit lesion and the patients’ medical comorbidities. Lesions of the supra-aortic trunks are more commonly associated with atherosclerosis and are most frequently approached via extrathoracic approaches, such as carotid-subclavian artery bypasses and transpositions. Transpositions are preferred over bypasses because the long-term patency is better.30,31 Intrathoracic reconstructions are generally avoided, especially with cardiopulmonary disease or a history of prior sternotomy, because the morbidity is markedly less for transpositions or bypasses31; however, if there is disease in the innominate artery or occlusive disease in multiple major vessels, transthoracic reconstructions based on the ascending aorta may be preferable.31,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree