CHAPTER 86 Surgical Treatment of Arrhythmias
ATRIAL FIBRILLATION
Background
Atrial fibrillation (AF) is present in up to 2% of the general population and in approximately 10% of patients older than 60 years, making it the most common form of sustained cardiac arrhythmia.1–5 Admissions for inpatient care associated with AF have risen dramatically in the last several years and are expected to continue to increase.6 AF annual hospitalizations are predicted to rise from 376,000 in 1999 to more than 3.3 million by 2025 in the United States.6
Although AF is frequently considered to be an innocuous arrhythmia, it can be associated with significant morbidity and mortality because of its three detrimental sequelae: (1) palpitations, which cause the patient discomfort and anxiety; (2) loss of synchronous atrioventricular contraction, which compromises cardiac hemodynamics, resulting in varying degrees of ventricular dysfunction; and (3) stasis of blood flow in the left atrium, which can result in thromboembolism and stroke.7–11 It has been estimated that AF results in a threefold to fivefold increase in a patient’s risk of stroke.12 Twenty percent to 30% of all acute stroke patients are found to be in AF.13–15 AF also independently increases mortality rates. Using data from the Framingham Heart Study, Benjamin and coworkers16 established the risk factor–adjusted odds ratio for death in men and women with AF as 1.5 and 1.9, respectively.
Classification of Atrial Fibrillation
There are several systems of classifying AF, but the classification system published jointly by the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society is the most widely used.17 This system defines AF as either paroxysmal or persistent. When a patient has had two or more episodes, AF is considered recurrent. If recurrent AF terminates spontaneously, it is designated paroxysmal; but if it is sustained beyond 7 days, it is termed persistent. Termination by pharmacologic therapy or electrical cardioversion before expected spontaneous termination does not change the designation persistent. In a recent consensus statement sponsored by the Heart Rhythm Society, the term permanent was changed to include only cases in which cardioversion has failed, clarifying that patients with long-standing AF might still be cured by intervention. In those cases, permanent has been replaced with the term long-standing AF when the duration is more than 1 year.18
Electrophysiology of Atrial Fibrillation
AF is characterized by the irregular activation of the atria and an accompanying irregular ventricular response. Activation of the atria during AF can exhibit two different patterns. One pattern consists of a stable source, either a focal trigger or a small reentrant circuit, with fibrillatory conduction away from the source. The other pattern is characterized by multiple changing sources or reentrant circuits. The specific mechanism may change over time in a particular patient. Work from our laboratory obtained from patients undergoing intraoperative mapping before arrhythmia surgery revealed that the source of AF was not stable in almost half of the patients, even moving from one atrium to the other.19
Four substrates determine whether AF is initiated and sustained: (1) a trigger, usually a premature depolarization or runs of focal ectopic depolarizations; (2) the refractory period of the atria and its magnitude and spatial distribution; (3) the conduction velocity and its magnitude and anisotropic spread; and (4) the geometry or anatomy, both macroscopic and microscopic. Whatever the pathologic process (e.g., valvular disease, heart failure, ischemia, tachycardia, pericarditis, inflammation), the changes that occur in the atrial myocardium and the physiologic changes affect one or more of these four factors.20
The surgical treatment of AF is directed at alteration of the geometry and anatomy needed to support AF. Non-reentrant mechanisms, such as abnormal automaticity and triggered activity, are important for the generation of premature beats, which act as a trigger for reentry but may also be involved in maintaining AF. The premature beats interact with the underlying distribution of refractory periods. As the distribution becomes more inhomogeneous, unidirectional block can occur. This is a necessary condition for the initiation of reentry. When unidirectional block occurs, a reentrant arrhythmia will occur only if a critical mass of tissue is present. The critical mass is determined by the tissue geometry, the magnitude of refractory periods, and the conduction velocity. The amount of tissue required to support a reentrant circuit is defined by the equation WL = CV × RP (wavelength = conduction velocity × refractory period). If either CV or RP decreases, the amount of tissue needed to sustain AF decreases, and the probability of a patient’s having an arrhythmia increases.21 Nonpharmacologic treatment approaches attempt to alter one of these substrates. However, any intervention has the potential to affect the other substrates that cause AF. For example, incisions or ablations not only affect conduction, but they alter the geometry of the atria, decrease viable myocardial mass, and can denervate regions of the atria, which alters refractory periods.21
A great deal of emphasis, 1972 to 2002 has been placed on the role of the pulmonary veins in triggering of AF. Paroxysmal AF often originates in the pulmonary veins.22 In humans, the anatomy of the pulmonary veins is variable, with electrically excitable cardiac muscle extending 1 to 4 cm beyond the ostium of the veins.23 Developmental biological studies suggest that pacemaker tissue may be present in the pulmonary veins.24 Another potential mechanism of focal activation is afterdepolarization.25 Intraoperative mapping studies have shown ectopic atrial beats originating from the region of the pulmonary veins.20 Biatrial mapping studies by Schmitt and coworkers26 have shown that the premature beats that trigger AF were located in the pulmonary veins 53% of the time and in the posterior atrium in another 29% of cases.
Successful cure of AF is achieved in some patients by the isolation of the pulmonary veins.22 Furthermore, if triggers of AF were outside the pulmonary veins but other substrates that sustain AF were within the veins, AF would be prevented with pulmonary vein isolation. The failure to cure AF by isolating only the pulmonary veins in some patients, especially those with long-standing AF, suggests that other anatomic triggers or more complicated mechanisms may be involved in these instances. Nitta and colleagues27 determined that there can be concurrent atrial focal activation as the mechanism of AF by use of intraoperative mapping in patients with mitral valve disease. Furthermore, caution should be taken in interpreting various interventional studies, whether catheter ablation or surgery, as to whether they imply an underlying mechanism involved in a patient’s AF. Most intraoperative and catheter mapping systems do not have the spatial resolution within the pulmonary veins to separate reentrant from non-reentrant mechanisms. Therefore, even though “focal” fibrillation may be reported from investigational mapping, this does not rule out reentry as a mechanism underlying the arrhythmia. Claims of cure by pulmonary vein isolation alone must be tempered by the knowledge that the “pulmonary vein isolation” intervention actually incorporates much more than just the pulmonary veins. Commonly, the pulmonary veins, the adjacent atrial muscle, and the muscle in the oblique sinus between the veins are ablated during a catheter-based pulmonary vein isolation. This area is more than one third of the left atrium. This large area of ablation substantially reduces the critical mass needed to sustain AF and may incorporate other non–pulmonary vein substrates of AF.
The definitions of paroxysmal, persistent, and long-standing AF do not imply a specific mechanism. Even though clinical results have shown that pulmonary vein isolation is effective 70% to 80% of the time in paroxysmal AF, it is clear that the pulmonary veins are not the only substrate driving AF 20% to 30% of the time.19 Likewise, in persistent AF, pulmonary vein isolation alone is successful in only a minority of patients.18 Human mapping data from our laboratory did not show any significant difference in mechanism between paroxysmal and persistent AF.19 Unfortunately, present diagnostic technologies rarely allow a preoperative delineation of mechanism. However, electrophysiologic studies may allow physicians to identify triggers of AF in some patients.19 Because AF is a complex arrhythmia, mapping requires a high density of closely placed electrodes as well as a sophisticated mapping and signal processing system to define the particular mechanism in an individual patient. Intraoperative mapping has not been useful in our hands in providing real-time information during surgery. The analysis of this complex arrhythmia is time-consuming and difficult. Therefore, the traditional surgical algorithm of obtaining preoperative or intraoperative mapping data and using this information to guide the specific surgical technique, as was done with arrhythmias such as Wolff-Parkinson-White syndrome, has not been feasible for AF. However, mapping techniques are currently being developed that may allow interventionalists to customize the incision set to the specific underlying mechanism.19,28–30
One particularly promising technique is electrocardiographic imaging.31 This allows AF to be mapped noninvasively in the awake patient by recording signals from the body surface and solving the inverse equation. This would delineate the mechanism before the proposed intervention and may allow physicians to triage patients to the most effective procedure.
Medical Treatment
Results with medical therapy alone for AF have been disappointing. Antiarrhythmic drugs have had limited long-term efficacy in converting AF to normal sinus rhythm and have significant and sometimes fatal side effects.32–37 The goal of pharmacotherapy is therefore often shifted from rhythm to rate control, that is, slowing the ventricular response rate to AF and avoiding the development of rate-related cardiomyopathy and symptoms such as palpitations. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study38,39 showed that management with rhythm control did not have any survival benefit over a rate control strategy in anticoagulated patients with AF.40,41 Furthermore, rate control strategy may potentially have advantages over rhythm control, such as a lower risk for adverse side effects that can be seen with aggressive rhythm control.40
Rate control alone clearly has disadvantages. Although the ventricular response rate can often be controlled pharmacologically, the atria are still in fibrillation. With persistent AF, two of the three detrimental sequelae associated with AF persist. In patients with baseline cardiac dysfunction, the absence of atrial “kick” often can result in worsening symptoms of congestive heart failure. Most important, patients with AF are at a risk for developing thromboembolism, requiring indefinite anticoagulation with warfarin. The use of warfarin itself is associated with a major complication rate of approximately 2% per year.42–44
Despite the results from the AFFIRM trial supporting no difference in long-term outcome between rhythm and rate control, there are many clinically meaningful advantages of being in normal sinus rhythm. These advantages include increased exercise tolerance, no need for anticoagulation medications, decreased palpitations, and prevention of atrial remodeling.39,45 Most important, in the AFFIRM trial, the presence of sinus rhythm was associated with a significantly decreased risk of death (hazard ratio = 0.53; P < .0001).46 Thus, the AFFIRM trial demonstrated that antiarrhythmic drugs are detrimental but that normal sinus rhythm is beneficial in this population of patients, suggesting a role for nonpharmacologic restoration of sinus rhythm.
Historical Aspects of Surgery for Atrial Fibrillation
Because of the inadequacy of medical therapy for AF, several procedures were developed in the 1980s aimed at treatment of AF. However, most of these were abandoned because of their inability to eliminate all three of the detrimental sequelae associated with AF.47–49 Nevertheless, they helped physicians gain fundamental knowledge about the mechanism of AF and laid a foundation for the development of the Cox-Maze procedure and its subsequent iterations. The Cox-Maze procedure today is recognized as the “gold standard” for surgical cure of AF. The next section briefly describes these various surgical procedures developed in an attempt to cure AF.
The Left Atrial Isolation Procedure
In 1980, Williams’ group developed the left atrial isolation procedure, which confined AF to the left atrium, thus restoring the remainder of the heart to normal sinus rhythm.49 This procedure had the advantage of restoring normal ventricular rhythm without requiring a permanent pacemaker. Because the sinoatrial node, atrioventricular node, and internodal conduction pathways are located in the right atrium and interatrial septum, the left atrial isolation procedure did not interfere with normal atrioventricular conduction.
Catheter Ablation of the Atrioventricular Node–His Bundle Complex
In 1982, Scheinman and colleagues50 described the catheter fulguration of the His bundle, which controlled the irregular cardiac rhythm associated with AF and other refractory supraventricular arrhythmias. Similar to the left atrial isolation procedure, this procedure electrically isolated the arrhythmia to the atria. However, ablation of the His bundle necessitated implantating a permanent ventricular pacemaker to restore normal ventricular rhythm.
The Corridor Procedure
In 1985, Guiraudon and associates51 introduced the corridor procedure for the treatment of AF. This was an operation that isolated a strip of atrial septum harboring both the sinoatrial node and the atrioventricular node, thereby allowing the sinoatrial node to drive the ventricles. This procedure corrected the irregular heart beat associated with AF, but both atria either remained in fibrillation or developed their own asynchronous intrinsic rhythm because they were isolated from the septal “corridor.” The atria were also isolated from their respective ventricles, thereby precluding the possibility of atrioventricular synchrony. The corridor procedure was soon abandoned because neither the hemodynamic compromise nor the risk of thromboembolism associated with AF was addressed.
The Atrial Transection Procedure
In 1985, Cox’s group described for the first time a series of experiments that attempted to cure AF in a canine model.52 After a number of experiments, it was found that a single long incision across both atria and down into the septum cured AF. This “atrial transection” procedure prevented the induction and maintenance of AF or atrial flutter in canines.54 Unfortunately, this procedure was not curative in its clinical application in humans.
Development of the Cox-Maze Procedure
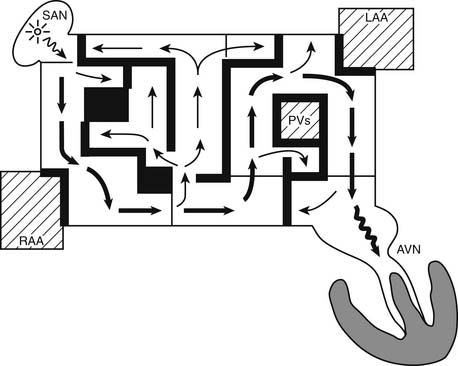
The surgical procedures that were developed for the treatment of arrhythmias led to the development of the Maze procedure by the team led by James L. Cox at Washington University in St. Louis in 1987.53–55 The Cox-Maze procedure was designed to interrupt the macro-reentrant circuits that were thought to be responsible for AF at that time, thereby precluding the ability of the atrium to flutter or fibrillate (Fig. 86-1). In contrast to previous procedures, the Maze procedure successfully restored both atrioventricular synchrony and a regular heart beat, thus decreasing the risk of thromboembolism and stroke.56 The operation involved creating myriad incisions across both the right and left atria. The surgical incisions were placed so that the sinoatrial node could “direct” the propagation of the sinus impulse throughout both atria. It also allowed all of the atrial myocardium to be activated, resulting in preservation of atrial transport function in most patients.57
The original technique, the Maze I procedure, was introduced in 1987, only to be soon modified to become the Maze II procedure because of late chronotropic incompetence and a high incidence of pacemaker implantations. The Maze II procedure, however, proved to be technically difficult to perform. It was therefore modified again to become the Maze III procedure (Fig. 86-2).58,59
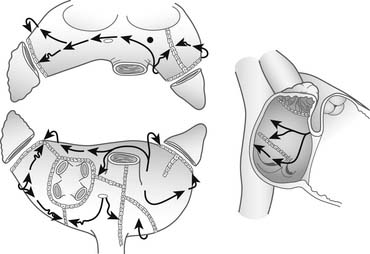
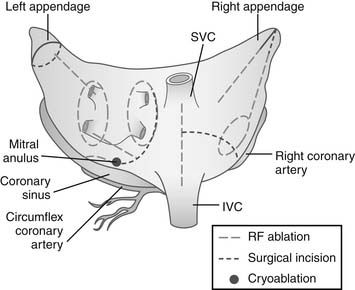
Figure 86–2 Two-dimensional drawing depicting the atrial incisions of the Cox-Maze III procedure.
(From Cox JL, Boineau JP, Schuessler RB, et al. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results J Thorac Cardiovasc Surg 1995;110:473–84.)
During the last decade, the Cox-Maze III procedure has become the gold standard for the surgical treatment of AF. In a long-term study of patients who had the Cox-Maze procedure, 97% of the patients at late follow-up were free of AF.60 Similar results have been reproduced by other institutions around the world.61–63
Surgical Ablation Technology
Despite its proven efficacy, the Cox-Maze III procedure did not gain widespread acceptance. Few cardiac surgeons were willing to add the procedure to a coronary revascularization or valve procedure because of its complexity and technical difficulty. In an attempt to simplify the operation and to make it more accessible to cardiac surgeons, groups around the world have replaced the incisions of the traditional cut- and-sew Cox-Maze III procedure with linear lines of ablation.64 These linear lines of ablation have been created by use of a variety of energy sources including radiofrequency energy, microwave, cryoablation, laser, and high-frequency ultrasound.
The development of these new ablation technologies has revolutionized the surgical treatment of AF by taking a technically difficult and time-consuming operation and making it relatively easy for all cardiac surgeons to perform. Whereas very few patients (<1%) with AF undergoing cardiac surgery before 2000 underwent a Cox-Maze procedure, a study has shown that more than 40% of patients with AF undergoing cardiac surgery had a concomitant ablation procedure in 2006.65 Another advantage of ablation technologies is the potential for the development of less-invasive operations. A minimally invasive beating heart procedure with high efficacy is the ultimate goal of these efforts. With the availability of easy-to-use ablation devices, numerous groups around the world have introduced a variety of new procedures for AF involving more limited sets of atrial lesions. Some groups are currently performing only the left atrial lesions, whereas others are advocating pulmonary vein isolation alone. Results with these new procedures are discussed in a later section.
For ablation technology to reliably replace the incision in AF surgery, it must meet several criteria. Foremost, it must reliably produce bidirectional conduction block. This is the mechanism by which incisions prevent AF: by blocking macro-reentrant or micro-reentrant circuits, by isolating trigger foci, or by reducing atrial contiguous mass. To do this with certainty, an ablation device must have the capability to reliably make transmural lesions from either the epicardial or endocardial surface. Experimental work has shown that even small gaps in ablation lines can conduct fibrillatory wavefronts.66
Cryoablation
The size and depth of cryolesions are determined by numerous factors, including probe temperature, tissue temperature, probe size, duration and number of ablations, and particular liquid used as the cooling agent.67 With conventional nitrous oxide, 2- to 3-minute ablations have been shown to reliably create transmural lesions on both the right and left atrium. Because of the heat sink provided by circulating endocardial blood, epicardial cryolesions on the beating heart have not been uniformly transmural.68 In one study, investigators were able to create transmural lesions 62% of the time around the pulmonary veins, and two of eight ablations (25%) on the left atrial appendage were transmural.68 However, in another study of cryoablation on the beating heart, consistent transmural lesions were created on tissue up to 7 mm thick.69 Ablations around the pulmonary veins were created, and acute electrical isolation was achieved in 13 of 13 animals. All except one of the animals maintained electrical isolation long term. Whereas histologic analysis revealed transmurality in 89% of sections, none of the box lesions around the pulmonary veins was completely transmural, again emphasizing the drawbacks of cryoablation on the beating heart.
Because of its ease of use and safety profile, many groups use cryoablation on the heart for the treatment of AF. A European randomized trial comparing mitral valve surgery alone with mitral valve surgery with left atrial ablations found an increase in cure for AF in the cryoablation group (freedom from AF at 12 months: 43% versus 73%).70
Cryoablation has the benefit of preserving the fibrous skeleton of the heart, making this an ideal choice for ablation near valvular tissue. Nitrous oxide cryoablation has had extensive clinical use and has had an excellent safety profile. Although cryothermal energy appears to have no permanent effects on valvular tissue or the coronary sinus, experimental studies have shown late intimal hyperplasia of coronary arteries, and these structures should be avoided.71–74 In the report by Doll and colleagues,68 they were able to create mild esophageal lesions in seven of the eight cases with epicardial cryoablation.
Cryoablation is unique among the currently available ablation technologies in that it destroys tissue by freezing rather than by heating. The important advantage is its ability to preserve tissue architecture. The nitrous oxide technology has a well-defined efficacy and safety profile and is generally safe except around the coronary arteries. The potential disadvantages of cryoablation technology include the relatively long time necessary to create a lesion (1 to 3 minutes). There is also difficulty in creating lesions on the beating heart because of the heat sink of the circulating blood volume. Furthermore, if blood is frozen during epicardial ablation on the beating heart, it coagulates, creating a potential risk of thromboembolism.
Radiofrequency Energy
Radiofrequency (RF) energy has been used for cardiac ablation for many years in the electrophysiology laboratory.75 It also was one of the first energy sources to be used in the operating room. RF energy can be delivered by either unipolar or bipolar electrodes, and the electrodes can be either dry or irrigated.
Bipolar RF is similar to unipolar energy except that two electrodes, instead of one, are used to focus the path of energy. This allows faster ablation (usually less than 10 seconds) while limiting destruction to tissue that is close to the electrodes. With bipolar devices, the electrodes are clamped over the targeted atrial tissue. The first bipolar RF device was introduced by AtriCure, Inc. The Isolator was a specially designed clamp with 1-mm-wide, 5-cm-long electrodes embedded in the jaws of the clamp. The device was unique in that it had an algorithm created to detect real-time measurement of lesion transmurality. The conductance between the electrodes was measured during ablation. When the conductance dropped to a stable minimum level, this correlated well both experimentally and clinically to histologically transmural lesions.76–78 More recent iterations have introduced more uniform clamp strength and a unique dual electrode designed to achieve wider and more consistent lesions.
Newer RF ablation devices have been released by other manufacterers.79,80 The Cobra Adhere and Cobra Adhere XL (Estech) devices are streamlined with suction stabilization for use in minimally invasive procedures, such as port access and thoracoscopic approaches. The Medtronic bipolar device, the Cardioblate BP, has an irrigated, flexible jaw along with an articulating head, with 5-cm-long electrodes. This device has an algorithm to predict transmurality of lesions similar to that of the AtriCure device and has also been shown to be effective in the experimental setting.81,82
RF energy uses an alternating current in the range of 100 to 1000 kHz. This frequency is high enough to prevent rapid myocardial depolarization and the induction of ventricular fibrillation, yet low enough to prevent tissue vaporization and perforation. Resistive heating occurs only within a narrow rim of tissue in direct contact with the electrode, usually less than 1 mm. The deeper tissue heating occurs by passive conduction. With unipolar catheters, the energy is dispersed between the electrode tip and an indifferent electrode, usually the grounding pad applied to the patient. In the bipolar clamp devices, alternating current is generated between two closely approximated electrodes, which results in a more focused ablation. The lesion size depends on the electrode-tissue contact area, the interface temperature, the current and voltage (power), and the duration of delivery. The depth of the lesion can be limited by char formation at the tissue-electrode interface because of the high temperatures (>100°C). To resolve this problem, irrigated catheters have been developed; this reduces char formation by keeping temperatures cooler at the tissue interface. These irrigated catheters have been shown to create larger volume lesions than the dry RF devices do.83,84
Dose-response curves for unipolar RF have been described.85–87 Although unipolar RF has been shown to create transmural lesions on the arrested heart in animals with sufficiently long ablation times (60 to 120 seconds), this has not been the case in humans. In one study, after 2-minute endocardial ablations during mitral valve surgery, only 20% of the in vivo lesions were transmural.86 Epicardial ablation has been even more difficult. Animal studies have consistently shown that unipolar RF is incapable of creating epicardial transmural lesions on the beating heart.87,88 One study in humans resulted in only 7% of lesions being transmural despite electrode temperatures of up to 90°C.89 Bipolar RF ablation, on the other hand, has been shown to be capable of creating transmural lesions on the beating heart both in animals and in humans with average ablation times between 5 and 10 seconds.76,77,90
Because RF ablation is a well-developed technology, much is known about its safety profile. A number of clinical complications of unipolar RF devices have been described, including coronary artery injuries, cerebrovascular accidents, and the devastating complication of esophageal perforation leading to atrioesophageal fistula.91–94 Use of the bipolar RF devices has eliminated virtually all of the collateral damage seen with the unipolar devices, and there have been no clinical complications reported in the literature. Unfortunately, the bipolar devices have the drawback of requiring that the tissue be clamped in the jaws of the device. This has limited the potential lesion set, particularly on the beating heart, and has required the use of adjunctive unipolar technology to create a complete Cox-Maze lesion set.
High-Intensity Focused Ultrasound
Although the interaction between ultrasound energy and biological tissue has been a subject of interest for decades, its application in cardiac tissue has only recently been studied.95–101 Ultrasound effectively ablates tissue through mechanical hyperthermia.102 When ultrasound waves are emitted from the transducer, the resulting wave travels through the tissue, causing compression, refraction, and particle movement. This translates into kinetic energy and ultimately thermal coagulative tissue necrosis.
Ultrasound can be used in either a focused or nonfocused form. High-intensity focused ultrasound (HIFU) produces a rapid, high concentration of energy and is able to create epicardial lesions through epicardial fat in less than 2 seconds.103 Nonfocused ultrasound, although it has a simpler transducer system and more flexibility, requires more time to create lesions.
HIFU has become an attractive modality for cardiac ablation for several reasons. It is unique in its ability to create noninvasive, noncontact focal ablation in three-dimensional volume without affecting intervening and surrounding tissue. HIFU uses ultrasound beams in the frequency range of 1 to 5 MHz or higher, creating focused lesions quickly by rapidly raising the temperature of the targeted tissue to above 80°C, effectively killing the cells.104 There is a steep temperature gradient between the focus and collateral tissue, demonstrated by the sharp demarcation seen between the volume of necrotic tissue and normal surrounding cells on histologic examination.105 By use of focused ultrasound waves, HIFU is able to create targeted thermal coagulation of tissue at a very well defined focus without harming intervening tissue, thus limiting collateral damage.
Another advantage of HIFU is its mechanism of thermal ablation. Most other energy sources transmit energy by thermal conduction, which creates a graded response dependent on the distance from the energy source. This makes them susceptible to cooling near blood vessels or heat sink effects.106,107 HIFU, on the other hand, ablates tissue by directly heating the tissue in the acoustic focal volume and is affected much less by the heat sink.108
Because ultrasound can be collimated through fluid medium, it is ideal for application with a balloon delivery system. Cummings and associates evaluated the use of an 8-MHz HIFU transducer mounted in a saline-filled balloon designed for pulmonary vein isolation for the treatment of AF. Unfortunately, only 30% of 33 patients who underwent pulmonary vein isolation were cured of AF in late follow-up. The variability of the pulmonary vein anatomy was the main reason for the poor results, often requiring several applications for pulmonary vein isolation to be achieved during the procedure.98,99
Because of its ability to focus the target of ablation at specific depths, epicardial surgical ablation by HIFU has gained interest. In a study by Ninet and coworkers,109 103 patients with AF underwent beating heart pulmonary vein isolation procedures with concomitant cardiac procedures.109 At a 6-month follow-up, the freedom from AF was 85%. Epicardial approaches using HIFU for the ablation of AF allow the possible development of minimally invasive techniques. Unfortunately, the excellent results have not been replicated by other groups. Moreover, the HIFU technology has not been tested experimentally by an independent laboratory to evaluate its effectiveness in creating transmural lesions in surgically relevant models.
Surgical Indications for Treatment of Atrial Fibrillation
The Heart Rhythm Society created a task force to evaluate indications for catheter and for surgical ablation of AF.18 The recommendations were developed in partnership with the European Heart Rhythm Association, the European Cardiac Arrhythmia Society, the American College of Cardiology, the American Heart Association, and the Society of Thoracic Surgeons. They recommended that programs involved in the stand-alone surgical treatment of AF should develop a team approach to these patients, including both electrophysiologists and surgeons, to ensure appropriate selection of patients. The consensus of the Task Force is that the following are appropriate indications for surgical ablation of AF: (1) symptomatic AF patients undergoing other cardiac surgical procedures and (2) selected asymptomatic AF patients undergoing cardiac surgery in whom the ablation can be performed with minimal risk. Stand-alone AF surgery should be considered for symptomatic AF patients who have failed medical management and prefer a surgical approach, have failed one or more attempts at catheter ablation, or are not candidates for catheter ablation.18
Other patients who should be considered for surgery include those who have developed a contraindication to warfarin or who have had a stroke while adequately anticoagulated, because the Cox-Maze procedure significantly reduces the risk of stroke in these patients. About 20% of patients who had the original cut-and-sew procedure at our institution had experienced at least one episode of cerebral thromboembolism that resulted in a temporary or permanent neurologic deficit before having the Cox-Maze procedure; less than 1% of patients (2 of 306) had a late stroke after surgery (mean follow-up of 11.5 years).110
Surgical Technique: Cox-Maze Procedure
The final version of the standard cut-and-sew technique to cure AF was the Cox-Maze III procedure.54,111–113 However, few cardiac surgeons performed this operation because of its technical complexity. At most centers, the majority of the surgical incisions have been replaced with linear lines of ablation by different energy sources. At Washington University, bipolar RF energy has been used successfully to replace the majority of the surgical incisions of the Cox-Maze III procedure. Our current procedure incorporates most of the lesions of the Cox-Maze III procedure and has been named the Cox-Maze IV (Fig. 86-3).114 Our clinical data have shown that this modified operation has significantly shortened the operative time while maintaining the high success rate of the traditional cut-and-sew Cox-Maze III procedure.115
Bipolar RF ablation was chosen for the Cox-Maze IV procedure for several reasons. First, the device allows the on-line determination of lesion transmurality by measuring the conductance between the two electrodes.86,116,117 Second, the bipolar RF device has extremely short ablation times, with 5- to 6-cm-long transmural ablations performed in 5 to 15 seconds. Third, the lesions are narrow (2 to 3 mm in width), and tissue injury is confined within the clamp. This eliminates the possibility of unwanted collateral injury.92,118–120
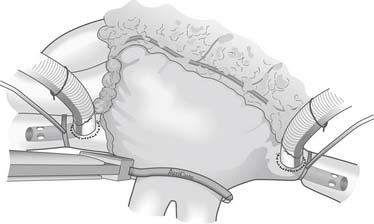
The Cox-Maze IV procedure is performed with the patient on cardiopulmonary bypass through either a median sternotomy or a right mini-thoracotomy. Initially, the heart is perfused at normothermia to maintain sinus rhythm and to allow accurate determination of pacing thresholds. The right and left pulmonary veins are bluntly dissected. If the patient is in AF, an intravenous bolus of amiodarone is given and the patient is cardioverted. The bipolar ablations are performed on the cuff of atrial tissue surrounding the right and left pulmonary veins (Fig. 86-4). Proof of electrical isolation is confirmed first by determining baseline pacing thresholds before application of the clamp and then by pacing from both the superior and inferior pulmonary veins after ablation.
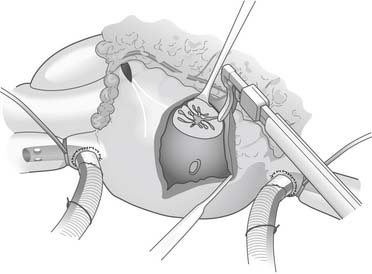
The right atrial lesions are performed with the heart beating. A small pursestring suture is placed at the base of the right atrial appendage. The bipolar clamp is placed through this to create the right atrial free wall lesion. A 2-cm gap is left between this ablation and a vertical atriotomy that extends from the crista terminalis up toward the atrioventricular groove near the acute margin of the right ventricle. The superior aspect of this incision is connected to the tricuspid anulus at the 2-o’clock position (Fig. 86-5). A unipolar energy source (e.g., cryoablation, RF) is used to complete this ablation line. From the pursestring at the base of the right atrial appendage, an ablation line is created across the right atrial endocardium down to the tricuspid anulus at the 10-o’clock position. To complete the right-sided lesions, the RF clamp is used to create lesions from the inferior aspect of the right atriotomy up to the superior vena cava and down toward the inferior vena cava (Fig. 86-6).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree