Introduction
Evidence-based management of patients with aortic valve disease is limited by the absence of prospective randomized trials of surgery versus medical therapy. Furthermore, the small number of prospective, randomized trials of medical treatment in aortic valve disease have provided conflicting results. However, retrospective studies provide extremely useful data which are important in the management of patients. Sir Thomas Lewis pointed out over 80 years ago the inadequacy of knowledge of prognosis in patients with heart disease and proposed a system for prospective follow-up of patients, which we now call “databases” or “registries”. The latter are, of course, the major evidence used in this chapter to delineate the indications for surgery and also influenced the recently updated American College of Cardiology (ACC) and American Heart Association (AHA) Guidelines for the Management of Patients with Valvular Heart Disease,1 and the European Society of Cardiology (ESC) Guidelines on the Management of Patients with Valvular Heart Disease2 which provide a framework upon which clinical decisions can be based. However, the guidelines must be put in perspective. The ACC/AHA guidelines had 124 pages and 1066 references. Of their 324 recommendations, only one (0.3%) was based on level of evidence A and 242 (74.7%) were based on level of evidence C. The ESC guidelines had 32 pages and 232 references. Of the 64 recommendations, none (0%) had level of evidence A and 58 (90.6%) were based on level of evidence C.
Etiology
A wide variety of disorders may produce aortic valve obstruction.3 However, those that result in severe stenosis in adults are:
- congenital
- acquired
- calcific (degenerative)
- autoimmune
- rheumatic.
The most common cause of aortic stenosis (AS) in younger adults is a congenital bicuspid valve, which is found in approximately 1% of the general population. In most patients aged 40–64 years, the severely stenotic aortic valve is bicuspid. In patients aged ≥65 years, 90% of severely stenotic valves are tricuspid. Non-rheumatic calcified valves are thought to be “degenerative” but recent data suggest that calcification and obstruction may be the result of an autoimmune reaction to antigens present in the valve,4 and that the initial process may be an atherosclerotic lesion.5,6 Early lesions are similar to atherosclerosis, with prominent accumulation of “atherogenic” lipoprotein, including low-density lipoprotein (LDL) and lipoprotein(a), evidence of LDL oxidation, an inflammatory cell infiltrate, and microscopic calcification. In late lesions, there are more prominent accumulation of lipid cells and extracellular matrix.7 Rheumatic heart disease is still common in developing countries.
Grading the degree of stenosis
The natural history of AS is variable depending on the degree of stenosis and the rate at which it progresses. Cardiac catheterization and echocardiographic –Doppler ultrasound studies indicate the systolic pressure gradient increases on an average by 10–15 mmHg per year. The 10–15 mmHg increase is not linear but a stepwise function with periods of steady state interspersed by an increase in gradient. The range of progression is also wide. Data suggest that the progression of AS may be related to cardiovascular risk factors8 and the rate of progression may be more rapid if marked leaflet calcification is noted by echocardiography.9 However, even some patients with only modest calcification may progress very rapidly, making careful follow-up essential.
The systolic gradient across the stenotic aortic valve is dependent on the following:
- the stroke volume (not the cardiac output because the gradient and valve area are a per beat, and not a per minute, function)
- the systolic ejection period
- the systolic pressure in the ascending aorta.
The stenotic valve area is inversely related to the square root of the mean systolic gradient. Thus, measurement of valve area is an important part of the assessment of the severity of AS. The valve area may decrease by as much as 0.12 ± 0.19cm2 per year.10
Valve area is related to body surface area and is larger in bigger individuals, probably because of the need for a larger stroke volume and cardiac output. The normal aortic valve area ranges from 3 to 4 cm2. It is reduced to half its size before a systolic gradient occurs.11 The orifice area has to be reduced to one-third of its size before significant hemodynamic changes are seen;12 gradients increase precipitously after that. The obvious clinical problem is that in an individual patient with AS, one usually does not know the valve area prior to the onset of disease.
Cardiac magnetic resonance imaging (MRI) and computed tomography (CT) are emerging tools in assessment of patients with AS.13,14 However, echocardiography remains the primary diagnostic tool and initial procedure used to confirm the presence and determine the severity of AS.15 In an experienced center the severity of AS determined by Doppler echocardiography correlates well with the severity determined by cardiac catheterization.16 A comprehensive echocardiographic-Doppler examination in AS should include assessment of the aortic valve peak and mean gradient as well as aortic valve area.17 When the clinical picture does not correlate with the hemodynamic data obtained by Doppler echocardiography, re-evaluation by cardiac catheterization is indicated.
Several series suggest B-type natriuretic peptide (BNP) is useful in assessing severity of aortic stenosis and identifying patients at high risk for cardiovascular events. It has been found that BNP is regulated by systolic and diastolic load, suggesting that myocardial stretch modulates BNP.18
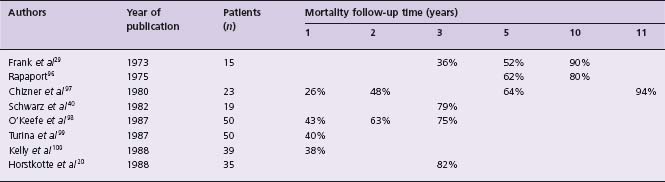
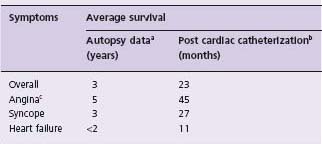
The outcome of patients with severe AS was described by Ross and Braunwald19 after review of seven autopsy studies published before 1955, and also by Horstkotte and Loogen20 reporting on 55 patients (10 of whom were asymptomatic) with aortic valve area of <0.8cm2 by cardiac catheterization who refused surgery. The findings are shown in Table 55.1. The mortality of symptomatic patients with “severe” AS from eight studies21 is given in Table 55.2.
Table 55.1 Survival, according to symptoms, of patients with “severe” aortic stenosis
a Data from Ross and Braunwald.19
b Data from Horstkotte and Loogen.20
c Angina in patients with AS occurs even in those without associated obstructive CAD.
Mild aortic stenosis
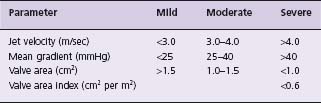
AS is best considered a disease continuum with resulting overlap of specific cut-off values. Nevertheless, a defined classification template is useful clinically. The ACC/AHA valve guidelines revised severity criteria1 are shown in Table 55.3. This document defines mild AS as an aortic valve area >1.5 cm2, peak aortic valve jet velocity by Doppler echocardiography <3.0m/sec, and mean gradient <25 mmHg. In two studies, patients with aortic valve area >1.5cm2 by catheterization had no mortality on follow-up. At the end of 10 years, in one study 8% had severe stenosis, and in the other 15% had a cardiac event. At the end of 20 years, AS had become severe in only 20% and continued to be mild in 63%.20,21
Moderate aortic stenosis
Moderate AS is defined as a valve area of >1.0–1.5cm2, peak aortic valve jet velocity by Doppler echocardiography 3.0–4.0m/sec, and mean gradient 25–40mmHg. In one study in which patients were followed after cardiac catheterization, the one-year and 10-year mortality was 3% and 15%, respectively, and at 10 years 65% of patients had had a cardiac event.21
Severe aortic stenosis
Several criteria have been used to define severe AS. The guidelines provided by the ACC and AHA Committee on Valvular Heart Disease1 describe severe AS as an aortic valve area <1.0cm2 and a mean aortic pressure gradient, in the setting of normal cardiac output, of >40mmHg. Although controversial, this definition was supported by data from a large multicenter database (492 patients) which suggested that the one-year mortality of those with aortic valve areas after catheter balloon valvuloplasty for calcific AS of <0.7cm2 versus that of those with valve areas >0.7cm2 1was 37% versus 42%, respectively22 Kennedy and co-workers23 reported on 66 patients with aortic valve areas of 0.7–1.2 cm2 (0.92 ± 0.15 cm2), normal left ventricular volumes and ejection fraction, whose average age was 67 years. In an average follow-up of 35 months, 21% died and 32% had valve replacement; at four years, the actuarial incidence of death or valve replacement was 41%.23 Thus, these studies show that patients with aortic valve areas of 0.7–1.0 cm2 have an outcome without valve replacement that is not benign, and is not consonant with moderate stenosis; these patients should be considered as having severe AS.
Natural history
The duration of the asymptomatic period after the development of severe AS is variable. Pellikka et al recently added to their previous work and reported the long term (5.4 ±4.0 years) follow-up of 622 patients (aged 72 ± 11 years) with asymptomatic, hemodynamically significant AS (peak jet velocity 4 m/sec by Doppler echocardiography).24 They found that most patients developed symptoms related to AS with the probability of remaining symptom-free at one, two and five years being 82%, 67%, and 33%, respectively. Furthermore, the one-, two- and five-year probabilities of remaining free of aortic valve surgery or cardiac death were 80%, 63%, and 25%, respectively with the risk of sudden death of approximately 1%/year. Patients having a peak velocity of >4.5 m/sec had a higher likelihood of developing symptoms (relative risk, 1.34) or having aortic valve surgery or cardiac death (relative risk, 1.48). In another study of 123 asymptomatic adults with varying grades of severity of AS (aged 63 ± 16 years),10 the actuarial probability of death or aortic valve surgery was 7 ± 5% at one year, 38 ± 8% at three years and 74 ± 10% at five years. The event rate at two years for aortic jet velocity by Doppler ultrasound of > 4.0 m/s (peak gradient by Doppler ultrasound >64mmHg) was 79 ± 18%, for a velocity of 3.0-4.0 m/s (peak gradient 36–64 mmHg) was 66 ± 13%, and for a velocity of < 3.0 m/s (peak gradient of <36mmHg) was 16 ± 16%.10 Thus, the duration of the asymptomatic period, particularly in those aged >60 years, is probably very short and the course of these patients is not benign.25,26 However, it should be remembered that it can be difficult to ascribe with certainty a cardiac cause of death if the patients died in their own communities far from the referral center.
Paul Dudley White in 195127 credited the first recorded occurrence of sudden death from AS to T. Bonet in 1679.28 In the past 70 years the reported incidence of sudden death in AS from eight series has ranged from 1% to 21%. Ross and Braunwald,19 after reviewing seven autopsy series published before 1955, concluded the incidence was 3–5%. The data reported by Pellikka et al24 suggest the risk of sudden death in asymptomatic patients with AS is approximately 1%/year. The incidence of sudden death in symptomatic adult patients has been 33% (one in three) in one series29 and 30% (three of ten) in another series.20 The development of symptoms of angina, syncope or heart failure changes the prognosis of the patient with AS. Average survival after the onset of symptoms is <2–3 years and careful clinical monitoring for the development of symptoms and progressive disease is paramount.
Management
Medical management
For many years, the standard of care has been to provide antibiotic prophylaxis against infective endocarditis in patients with significant AS.1 However, recently published AHA guidelines for the prevention of infective endocarditis have created significant controversy and no longer recommend antibiotic prophylaxis for AS based on lack of proven efficacy, risk of adverse reactions and poor cost-effectiveness unless the patient has had prior endocarditis, has a prosthetic valve, or has additional complex cardiac lesions that are high risk for the development of endocarditis.30 Patients who have had rheumatic fever should still receive antibiotic prophylaxis against recurrences of rheumatic fever1,31 (Class I, Level B; recommendation classes and levels of evidence are as noted in Box 55.1).
Unfortunately, there are no medical treatments proven to delay the progression of AS. AS shares similar atherosclerotic risk factors with coronary artery disease (CAD). Retrospective, observational studies have suggested that both statin therapy and possibly angiotensin-converting enzyme inhibition (ACEI) are effective in slowing progression of AS.32–35 However, no prospective trials assessing the efficacy of ACEI in AS exist and the two largest prospective, randomized trials looking at statin therapy in this setting have yielded conflicting results.36,37 Thus, further prospective trials with longer follow-up and assessment of these treatment strategies in mild or moderate AS are needed. Evaluation and modification of cardiovascular risk factors to prevent concurrent CAD as recommended by current guidelines is the best approach for now.
BOX 55.1 Recommendation classes and levels of evidence
Class I: Conditions for which there is evidence and/or general agreement that a given procedure or treatment is useful and effective.
Class II: Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment. Class IIa: Weight of evidence or opinion is in favor of usefulness/efficacy.
Class IIb: Usefulness/efficacy is less well established by evidence/opinion.
Class III: Conditions for which there is evidence and/or general agreement that the procedure/treatment is not useful, and in some cases, may be harmful.
Level of Evidence A: Data derived from multiple randomized clinical trials or meta-analyses
Level of Evidence B: Data derived from a single randomized clinical trial or large non-randomized studies
Level of Evidence C: Consensus of opinion of the experts and/or small studies, retrospective studies, registries
Surgical management
Surgery is recommended for severe symptomatic AS and is the only specific and direct therapy for most adults with severe AS. Rarely, in young patients, the aortic valve is suitable for balloon or surgical valvotomy. Recently, the results of transcatheter implantation of a balloon-expandable stent valve using a femoral arterial approach in 50 patients with severe AS at high risk for conventional open heart surgery have been published.38 The procedure was successful in 86% of patients and 30-day mortality was 12% in this high-risk cohort. With further refinement, percutaneous valve replacement may become an alternative to conventional open heart surgery in selected high-risk patients with severe symptomatic AS but in most adults, surgery for AS means aortic valve replacement (AVR).31,39 AVR is indicated for symptomatic patients with severe AS (Class I, Level B), although there are no prospective randomized trials of surgery versus no surgery in severe symptomatic AS, and there are unlikely to be any in the future.
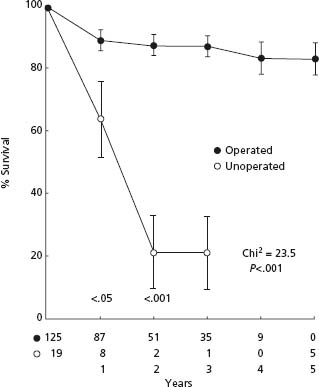
In severe symptomatic AS, AVR results in an improvement of survival even with normal preoperative left ventricular function.20,40 Schwartz et al39 compared the results of AVR with medical treatment during the same time period in patients with severe symptomatic AS and normal LV systolic function at a single center. They demonstrated significant survival benefit with AVR (Fig. 55.1). Horskotte and Loogen further demonstrated the poor prognosis of medical treatment in severe symptomatic AS with a mean survival of only 23 ± 5 months and a five-year probability of survival of only 18 ± 7% in patients who refused AVR.20 In patients who refused surgery, mean survival after the occurrence of angina pectoris was 45 ± 13 months, after syncope 27 ± 15 months, and after first occurrence of left heart failure 11 ± 10 months.20 These differences in survival between those treated medically and surgically are so large that there is a great deal of confidence that AVR signifi-cantly improves the survival of those with severe, symptomatic AS (Class I, Level B).
Figure 55.1 Aortic valve replacement is associated with a survival benefit in patients with severe symptomatic aortic stenosis. (From Schwarz et al40 with permission.) Open circles, unoperated; closed circles, operated.
The operative mortality of valve replacement is ≤5%.39,41,42 Data from the Euro Heart Survey demonstrate that operative mortality rates have dropped to 2.7% and 4.3%, for AVR and AVR+ coronary bypass surgery, respectively.43 Furthermore, in patients without other co-morbid conditions, the risk of AVR is ≤2% in experienced and skilled centers.44 The operative mortality in those ≥70 years is increased, averaging 8% for AVR and 13% for those undergoing AVR and associated coronary bypass surgery;39 however, operative mortality is also dependent on co-morbidities.45 Patients with associated CAD should have coronary artery bypass grafting at the same time as AVR, because it results in a lower operative mortality (4.0% vs 9.4%) and better 10-year survival (49% vs 36%).44 This was in spite of the fact that those who underwent coronary bypass surgery had more CAD (34% had three-vessel disease, 11% had left main artery disease, and 38% had single-vessel disease) than those who did not undergo coronary bypass surgery (13% had three-vessel disease, 1% had left main disease, and 65% had single-vessel disease).44 Although this approach to CAD is generally approved, there are no randomized trials to support these recommendations. The presence of CAD, its site and severity can be estimated best by selective coronary angiography, which should be performed in all patients 35 years of age or older who are being considered for aortic valve surgery, and in those aged <35 years if they have left ventricular dysfunction, symptoms or signs suggesting CAD, or they have two or more risk factors for premature CAD (excluding gender).39 The incidence of associated CAD will vary considerably depending on the prevalence of CAD in the population;21,39 in general, in persons 50 years of age or older it is about 50%.39 Normal preoperative left ventricular function remains normal postoperatively if perioperative myocardial damage has not occurred.46 Left ventricular hypertrophy regresses toward normal;46,47 after two years, the regression continues at a slower rate up to 10 years after AVR.47 In patients with excessive preoperative left ventricular hypertrophy,48 the hypertrophy may regress slowly or not at all. Preoperatively, these patients have a small left ventricular cavity, severe increase in wall thickness, and “supernormal” ejection fraction; this occurs in 42% of women and 14% of men aged ≥60 years.48 After AVR their clinical picture often resembles that of hypertrophic cardio-myopathy without outflow obstruction, which is a difficult clinical condition to treat, both in the early postoperative period and after hospital discharge;48 therefore, surgery should be performed prior to development of excessive hypertrophy. Surviving patients are functionally improved.39
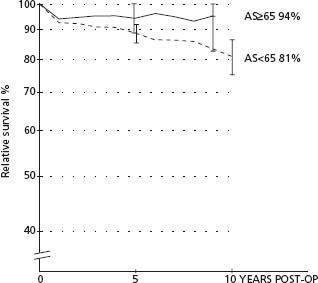
After AVR, the 10-year survival is ≥60% and 15-year survival is about 45%.39,49 One half or more of the late deaths are not related to the prosthesis but to associated cardiac abnormalities and other co-morbid conditions.49 Thus, the late survival will vary in different subgroups of patients. The older patients (≥60 years) have a 12-year actuarial survival of ≥60%.50 Data from the Karolinska Institute in Sweden have provided an interesting perspective on the long-term survival after AVR in patients with AS aged ≥65 years. They compared the relative survival of the patient who has undergone AVR with another age-and sex-matched person in the same population. Relative survival refers to survival of patients compared to age-and gender-matched people in the population. Patients under the age of 65 had a relative survival of 81% which is significantly lower than 100%, and is also lower than that of those aged ≥65 years. On the other hand, patients who underwent AVR at age ≥ 65 had a relative survival of 94% at the end of 10 years and this was not significantly different from 100% (Fig. 55.2). These data indicate that survival following AVR for AS in patients aged ≥65 is not significantly different from age-and sex-matched individuals in the population without AS; and the late relative survival of patients aged ≥65 years is much better than that of patients aged <65. Thus, surgery should not be denied to those ≥60–65 years old and should be performed early.50–52
Figure 55.2 Relative survival rates for patients operated on for pure AS. Patients are stratified according to age: <65 years old (dashed line) or ≥65 years old (solid line) with vertical bars indicating 95% confidence limits. Patients aged ≥65 who underwent AVR for AS obtained a normalized survival pattern after operation. POST-OP, postoperatively; AS, aortic stenosis; AVR, aortic valve replacement. (From Lindblom et al51 with permission.)
Patients who present with heart failure related to AS should undergo surgery as soon as possible. Medical treatment in hospital prior to surgery is reasonable to improve the perioperative risk, but ACE inhibitors should be used with great caution and in such a dosage that hypotension is avoided. Selected critically ill patients with left ventricular systolic dysfunction and severe AS may benefit from cautious administration of sodium nitroprusside therapy.53 However, this study is problematic. The goal was to study cardiac output by the Fick principle after 24 hours, but O 2 consumption was not measured but obtained from a nomo-gram that has no data on O 2 consumption in heart failure before and after treatment. The patients were made hypotensive and the one-month mortality was 25%. We strongly discourage the use of nitroprusside unless the patient is very hypertensive and needs intravenous therapy for control of hypertension and with the caveat that hypotension must be avoided. If heart failure does not respond satisfactorily and rapidly to medical therapy, surgery becomes a matter of considerable urgency39 Catheter balloon valvuloplasty has a very limited role in adults with calcific AS and carries a mortality risk of > 10%. In addition, restenosis and clinical deterioration occur within 6–12 months. In adults with AS, balloon valvuloplasty is not a substitute for AVR but can be a bridge procedure in selected patients.54 It may improve hemodynamics and make them better candidates for AVR. The results of valve replacement in patients with severe AS and preoperative left ventricular systolic dysfunction and the factors predictive of reduced postoperative survival, persistent left ventricular systolic dysfunction, and persistence of symptoms are summarized in Boxes 55.2 and 55.3.21,39,40,45–47,49–51,55–57
Surgical mortality for AS patients with heart failure has declined: 25 years ago the operative mortality was <20%57 but in the current era it is ≤10%.55 Although this is higher than in patients without heart failure, late outcome in those who survive the operation is excellent and is far superior to that which can be expected with medical therapy. Patients who survive operation have an 84% seven-year survival.56 Survival in those without associated CAD is greater than in those with CAD (69% vs 39% five-year survival, P = 0.02).55 Left ventricular function improves in most patients after AVR, and becomes normal in two-thirds of the patients, unless there was irreversible preoperative myocardial damage (Fig. 55.3).55,57 In addition, operative survivors are functionally much improved, and left ventricular hypertrophy and left ventricular dilation, if present preoperatively, regress toward normal.57 Despite the excellent results of AVR in patients with severe AS who are in heart failure, these results are not as good as for those who are not in heart failure; therefore, it is important to recognize that surgery should not be delayed until heart failure develops (Class I, Level B).
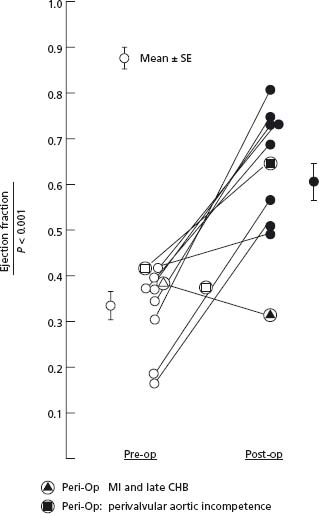
Figure 55.3 Change in LV EF for each individual patient with severe AS and LV systolic dysfunction. After AVR the LV EF improved from 0.34 to 0.63. All but one patient showed an improvement in LV EF; the only patient who showed deterioration in EF suffered a perioperative MI and had a complete heart block; and the only patient who showed only a relatively small increase in EF had had a MI prior to AVR. Note that LV EF normalized in two-thirds of the patients and in the two patients with the lowest ejection fraction (0.18 and 0.19), LV EF normalized in both. These data indicate that there is probably no lower limit of EF at which time patients with AS become inoperable. This also indicates that the lower the EF, the more urgent the need for AVR. AS, aortic stenosis; LV, left ventriclular; E F, ejection fraction; AVR, aortic valve replacement; MI, myocardial infarction. (From Smith et al57 with permission.)
BOX 55.2 Results of valve replacement in patients with severe aortic valve stenosis
- Improved symptoms and survival in symptomatic patients, especially in those with left ventricular systolic dysfunction, clinical heart failure, and in those aged ≤65 years
- Improvement in left ventricular systolic dysfunction, which normalizes in two thirds of patients
- Regression of left ventricular hypertrophy
- Improvement in functional class, more marked in those with severe symptoms preoperatively
BOX 55.3 Factors predictive of a less favorable outcome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree