Introduction and historic perspective
Throughout its development, coronary artery bypass grafting (CABG) has been studied extensively through randomized trials, prospective registries, and retrospective studies. It is arguably one of the most studied procedures in medicine. Despite improvements in medical therapies and percutaneous interventions, CABG remains an important therapy for millions of patients every year. Pioneering methods to restore blood supply to ischemic myocardium included placement of a pectoralis muscle flap on abraded pericardium by Beck in 1935,1 direct implantation of the internal thoracic artery (ITA) onto the myocardium in 1950 by Vineberg,2,3 and “myocardial acupuncture” in 1965 by Sen.4 Direct revascularization was possible after the development of the cardiopulmonary bypass (CPB) machine by Gibbon5 which, along with cardioplegia, allowed the heart to be stopped and the coronary arteries opened safely. In 1958, Longmire reported an ITA to coronary anastomosis after an unsuccessful coronary atherectomy.6 Goetz performed the first successfully planned CABG using a metal connector in 1960, with angiographic patency confirmed two weeks after operation.7 The first saphenous vein was used as a graft in 1962 by Sabiston.8 Kolessov, a Russian surgeon, was credited with the first successful planned sutured ITA to coronary artery anastomosis in 1964.9
After the development of coronary angiography at the Cleveland Clinic in 1957, the elective treatment of coronary atherosclerosis began to grow.10 The first large-scale series of CABG procedures were published between 1968 and 1970 demonstrating the success of CABG and ensuring its place in the treatment of coronary artery disease (CAD).11–15 Over the ensuing three and a half decades, the procedure has been refined with a decline in mortality rate below 2.5% currently.16 Curiously, one of the most recent developments is a return to “off-pump” techniques obviating the need for CPB, the very technologic innovation arguably most responsible for the widespread adoption of CABG.
Incidence, natural history, and prognosis
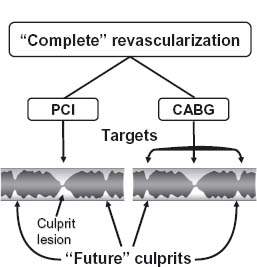
Given the clinical importance of the condition, the incidence, natural history, and prognosis of CAD in general are covered in detail elsewhere in this volume. Still, it is worth emphasizing that in contrast to percutaneous techniques, surgical revascularization has been definitively shown to improve five-year survival among patients with multivessel disease.17–19 This may be due to the ability of CABG to protect against future culprit lesions (Fig. 27.1).20
Figure 27.1 PCI is targeted at a specific culprit lesion. CABG protects against future culprit lesions by bypassing much of a coronary artery including both culprit and “future culprit” lesions. The dark shading indicates the residual lumen. (Reproduced with permission from Elsevier Ltd. Opie LH, Commerford PJ, Gersh BJ. Lancet 2006; 367:69–78.)
Coronary artery bypass grafting versus medical management
The American Heart Association and American College of Cardiology have developed comprehensive guidelines (Box 27.1) describing the indications for CABG based on patient symptom status and angiographic features of CAD.21 These guidelines are based on the best available evidence; however, many of the classic studies have limitations. Most patients in the CABG versus medical management trials were younger than 65 years of age, 80% had a left ventricular (LV) ejection fraction of ≥ 50%, and the majority of patients were men. Thus, patients enrolled in these trials were generally low risk from the standpoint of both the risk of performing the procedure and the risk of the natural history of the disease itself. Furthermore, the surgical patients did not routinely receive arterial bypass conduits nor did they receive routine aspirin postoperatively, both of which may be expected to positively impact graft patency. Of course, they also underwent surgical procedures without current myocardial protection strategies which may be expected to reduce perioperative risk. Equally, it must be said that among the patients who received medical therapy alone, there was no routine aspirin, beta-blockade or lipid-lowering therapy, nor were angiotensin-converting enzyme (ACE) inhibitors routinely prescribed. Despite these major limitations, these studies are remarkably consistent in their primary findings and their conclusions continue to be supported by recent studies, suggesting that they remain generalizable to current practice.
The Coronary Artery Surgery Study (CASS),22 the Veterans Administration (VA) Co-operative Study Group,23,24 the European Coronary Surgery Study (ECSS),25,26 and several other smaller randomized trials27–30 conducted between 1972 and 1984 provide outcome data comparing medical and surgical therapy. The relative benefits of CABG over medical therapy on survival are greatest in patients at highest risk of complications of their disease as defined by the severity of angina and/or ischemia, the number of diseased vessels, and the presence of LV dysfunction31 (Fig. 27.2). The survival advantage of surgery over medicine has not been demonstrable among patients with single-vessel disease.32–34 It should be emphasized, however, that these trials involved primarily patients with moderate chronic stable angina. These conclusions may, therefore, not necessarily apply to patients with unstable angina or to patients with more severe degrees of chronic stable angina in whom the area of myocardium at risk may be higher.
Figure 27.2 After 10 years of follow-up, CABG extends survival in most moderate-and high-risk subgroups when compared to medical management. Patients were classified into low, moderate, and high risk using the formula: (0.015 × age) + (0.56 × presence of class III/IV angina) + (0.35 × history of myocardial infarction) + (0.62 × abnormal ejection fraction) + (0.53 × proximal lesion of > 50% in LAD). (Reproduced with permission from Elsevier Ltd. Yusuf S, Zucker D, Passamani E et al. Lancet 1994;344:563–70.)
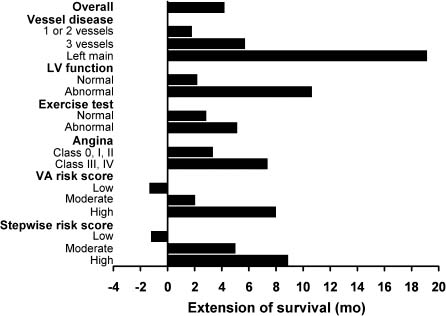
A meta-analysis31 of the seven randomized trials cited above demonstrated a survival benefit at five, seven and 10 years for surgically treated patients at highest risk of death due to their disease and among those at moderate risk, but no evidence of a survival benefit for those patients at lowest risk, as defined by a modified version of the Veterans Administration Risk Score23 and a stepwise risk scoring system.31 These scoring systems used a combination of both clinical and angiographic variables including angina class, history of hypertension, history of myocardial infarction, depressed ejection fraction (< 50%), and a proximal left anterior descending (LAD) lesion >50%. Non-randomized studies have also demonstrated a beneficial effect of surgery on survival of patients with multivessel disease and severe ischemia regardless of LV function.35–38
The more recent randomized trials of CABG versus medical therapy also support a strategy of revascularization among patients with symptomatic angina. The Trial of Invasive versus Medical therapy in Elderly (TIME) enrolled elderly (> 70 y) patients with chronic symptomatic CAD. The incidence of major adverse events (death, non-fatal myocardial infarction (MI) or hospitalization) was lower (19% vs 49%, P < 0.0001) and angina relief was greater (anginal class decreased by 2 vs 1.3, P < 0.0001), and all measures of quality of life (P < 0.05 for SF36 score, Duke activity score, and Rose score) were superior for those undergoing coronary angiography and surgical revascularization than in those randomized to an initial trial of medical therapy without angiography.39 In the Asymptomatic Cardiac Ischemia Pilot (ACIP) trial, patients with anatomy amenable to CABG were randomized to anti-ischemic therapy directed at relief of angina, to drug therapy guided by non-invasive measures of ischemia, or to revascularization by CABG or percutaneous coronary interventions (PCI).40 At two years, mortality was 6.6% in the angina-guided group, 4.4% in the ischemia-guided group, and 1.1% in the revascularization group (P < 0.02). At two years of follow-up, the rates of death or MI were 12.1%, 8.8%, and 4.7% respectively (P < 0.04). In the recent BARI 2D trial performed in patients with type 2 diabetes and stable coronary artery disease, by 5 years of follow-up there were fewer major cardiovascular events among CABG patients than among those receiving only optimal medical therapy.40a
Early concern over high operative mortality in patients with decreased LV systolic function has been replaced by the realization that the survival benefit to these patients is particularly great with surgical revascularization compared with medical therapy. Thus, LV dysfunction in patients with documented ischemia is now considered an important indication for CABG21,22,35,41,42 with operative mortality rates as low as 4.6%.43 Evidence that hibernating or stunned myocardium regains contractile function following effective revascularization44,45 has prompted expansion of the indications for surgical revascularization among patients with severe LV dysfunction to encompass most patients, including those who would otherwise be considered candidates for cardiac transplantation.
BOX 27.1 American College of Cardiology/American Heart Association Guidelines published in 2004 outline the indications for coronary artery bypass grafting.21 This box summarizes guidelines for asymptomatic or mild angina and stable angina.
Asymptomatic or mild angina
Class I
- Significant left main coronary artery stenosis. (Level A)
- Left main equivalent: ≥70% stenosis of the proximal LAD and proximal left circumflex artery. (Level A)
- Three-vessel disease. (Survival benefit is greater in patients with abnormal LV function, e.g. LVEF < 50% and/or large areas of demonstrable myocardial ischemia.) (Level C)
Class IIa
- Proximal LAD stenosis with one- or two-vessel disease. (Becomes a Class I if extensive ischemia is documented by non-invasive study and/or LVEF <50%.) (Level A)
Class IIb
- One- or two-vessel disease not involving the proximal LAD. (If a large area of viable myocardium and high-risk criteria are met on non-invasive testing, this recommendation becomes Class I.) (Level B)
Stable angina
Class I
- Significant left main coronary artery stenosis. (Level A)
- Left main equivalent: ≥70% stenosis of the proximal LAD and proximal left circumflex artery. (Level A)
- Three-vessel disease. (Survival benefit is greater when LVEF <50%.) (Level A)
- Two-vessel disease with significant proximal LAD stenosis and either LVEF < 50% or demonstrable ischemia on no-ninvasive testing. (Level A)
- One- or two-vessel CAD without significant proximal LAD stenosis but with a large area of viable myocardium and high-risk criteria on non-invasive testing. (Level B)
- Disabling angina despite maximal non-invasive therapy, when surgery can be performed with acceptable risk. If angina is not typical, objective evidence of ischemia should be obtained. (Level B)
Class IIa
- Proximal LAD stenosis with one-vessel disease. (This recommendation becomes Class I if extensive ischemia is documented by non-invasive study and/or LVEF <50%). (Level A)
- One- or two-vessel CAD without significant proximal LAD stenosis but with a moderate area of viable myocardium and demonstrable ischemia on non-invasive testing. (Level B)
Class III
- One- or two-vessel disease not involving significant proximal LAD stenosis, patients who have mild symptoms that are unlikely to be due to myocardial ischemia, or who have not received an adequate trial of medical therapy and have only a small area of viable myocardium or have no demonstrable ischemia on non-invasive testing. (Level B)
- Patients with angina who have borderline coronary stenoses (50 – 60% diameter in locations other than the left main coronary artery) and no demonstrable ischemia on non-invasive testing. (Level B)
- CABG is not recommended for patients with stable angina who have insignificant coronary stenosis ( <50% diameter reduction). (Level B)
Apart from affording a survival benefit, CABG is indicated for the relief of angina pectoris and improvement in quality of life. Between 80% and 90% of patients who are symptomatic on medical therapy become symptom free following CABG. This benefit extends to low-risk patients for whom survival benefit from surgery is not likely.31 Relief of symptoms appears to relate to both the completeness of revascularization and maintenance of graft patency, with the benefit of CABG diminishing with time. Recurrence of angina following CABG surgery occurs at rates of 3–20% per year.46–49 Although enhanced survival is reported when an ITA graft is used to the LAD, there is no significant difference in postoperative freedom from angina when compared to venous grafts.50 This may be due to occurrence of ischemia due to diseases in the vein grafts used to targets other than the LAD or ischemia due to progression of native disease.
Unfortunately, few patients experience an advantage in work rehabilitation with surgery as compared with medical management. Generally, employment declines in both groups and is determined nearly as much by socioeconomic factors such as age, preoperative unemployment, and type of job, as by type of therapy or clinical factors such as postoperative angina.51–53 Notably, surgical revascularization has not been shown to reduce the incidence of non-fatal events such as MI, although this may be due to perioperative infarctions which offset the lower incidence of infarction in follow-up.36,54
Coronary artery bypass grafting versus percutaneous interventions
This topic is discussed in Chapter 28.
On-pump and off-pump coronary artery bypass grafting
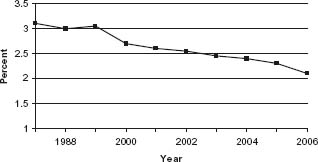
The current results of conventional on-pump CABG (ONCAB) are excellent. Perioperative mortality rates as reported to the Society of Thoracic Surgeons (STS) database have demonstrated a consistent decline despite an increasing patient risk profile (Fig. 27.3).55 In 2005, the unadjusted mortality rate for isolated primary CABG in the STS database was 2.2% and the median hospital length of stay was only five days.16 In return, the operation provides durable relief from angina pectoris and improves long-term survival.
Figure 27.3 The Society of Thoracic Surgeons database demonstrates a decreasing unadjusted operative mortality for patients undergoing isolated CABG procedures. This is despite increasing numbers of high-risk patients undergoing CABG. http://www.sts.org/sections/stsnationaldatabase/publications/executive/article.xhtml.
ONCAB comes at a physiologic price, however, as CPB causes trauma to red cells and platelets while inducing a generalized inflammatory response and activation of the cytokine, coagulation, and fibrinolytic cascades.56 Non-pulsatile flow may have adverse effects on end-organ function57 and cannulation for arterial inflow may cause embolization of either air or calcium. Despite these risks, ONCAB became the predominant method of surgical revascularization through the 1980s.58
While the techniques and technologies to perform ONCAB were being refined, including the introduction of biocompatible surfaces and centrifugal pumps,59 interest in off-pump (OPCAB) techniques persisted among a small number of surgeons. In the early 1990s, observational studies were published60–63 demonstrating good outcomes using OPCAB techniques. These data, along with a drive to make procedures less invasive by avoiding the known adverse effects of CPB, stimulated the development of cardiac stabilizing and positioning devices which have simplified OPCAB procedures.
Unfortunately, despite initial enthusiasm, it has been difficult to prove unequivocal superiority of OPCAB to ONCAB. As a general statement, retrospective studies have tended rather consistently to demonstrate benefit of OPCAB, while prospective randomized studies have not. This may be in part due to the tendency of randomized trials to enroll low-risk patients and observational studies to include higher-risk patients. When mainly low-risk patients are enrolled, demonstration of differences between any two therapies–OPCAB vs ONCAB or PCI vs CABG–becomes very difficult, as adverse events are infrequent and large numbers of patients are required for differences between treatments to reach statistical significance. Statistically significant differences are more likely to be demonstrable in observational trials thanks to the inclusion of high-risk patients.
Differences in mortality have been demonstrated by some investigators but not others. Randomized trials such as Octopus,64,65 SMART,66,67 PRAGUE-4,68,69 and others70-73 found no difference in survival. In an observational study of more than 68 000 patients, Racz and colleagues found no difference in risk-adjusted mortality between OPCAB and ONCAB.74 In a large propensity-matched cohort, however, Mack et al demonstrated a difference in adjusted mortality (2.2% vs 3.7%, P < 0.001) favoring OPCAB.75 Apparent differences in mortality have also been demonstrated among specific high-risk subgroups. In a retrospective study of patients with a low ejection fraction (<30%), OPCAB had a lower risk-adjusted mortality rate (1.47% vs 4.13%; P < 0.001).76 Puskas et al demonstrated that OPCAB was associated with a significant reduction in death (odds ratio (OR) 0.39, P = 0.001) when compared to ONCAB in a cohort of 7740 women.66,67 An important subgroup of patients with CAD are those with renal failure. In an analysis of patients with end-stage renal disease (ESRD), OPCAB was associated with an operative mortality of 1.7%, whereas the mortality rate of ONCAB was 10-fold higher (17.2%, P = 0.003).77 At late follow up, however, the OPCAB group of patients with ESRD had an increased late mortality when compared to the ONCAB group (38.1% vs 19% annual mortality, P = 0.03). This somewhat surprising result may be due to the lower number of mean grafts per patient in the OPCAB group (2.4 ± 1.0 vs 3.3 ± 0.9, P < 0.001).77
Apart from mortality, a number of retrospective studies have shown a benefit to OPCAB with regard to morbidity, including lower need for blood products, less reoperation for bleeding, and less renal failure,74,75 although the results of randomized trials have varied. Puskas reported results of the SMART study in 2003, in which patients randomized to OPCAB received fewer units of blood and cardiopulmonary bypass was an independent predictor of blood transfusion (OR 2.42, P = 0.007).66 The reduction in blood transfusion rates was also reported in both the BHACAS trial and by Khan et al72 but was not found to be different between OPCAB and ONCAB in other studies.69,70 In the SMART trial, postoperative length of stay was shorter in the OPCAB group by one day (P = 0.005)66 but in both the PRAGUE-4 study68,69 and others,70,72,73 no differences in hospital stay were observed between OPCAB and ONCAB.
There has been heightened awareness of neurocognitive changes occurring after CABG surgery. While this is a “soft” endpoint and defined by complex neurocognitive testing, the degree of public concern is justifiably great. It is arguable that a reduction in either neurologic decline or stroke would be the greatest benefit of OPCAB surgery. Unfortunately, this has been difficult to prove. A small randomized study demonstrated a dramatic superiority in cognitive outcomes at one week after OPCAB surgery78 Conversely, a randomized study of 60 patients demonstrated no difference in cognitive function after three months in OPCAB and ONCAB patients.79 The largest prospective randomized study performed to address this question was the Octopus Study which enrolled 281 low-risk patients randomized to OPCAB or ONCAB. There was no difference in cognitive outcomes at five years after surgery (P > 0.99).64,65 Interpretation of these data is made more difficult by the variability in neurocognitive testing, the impact of other factors on testing outcomes such as depression, and the anticipated decline in neurocognitive function with aging, particularly among patients with advanced atherosclerotic disease. Indeed, similar changes in neurocognitive function have been identified after PCI.80 Furthermore, the majority of individuals demonstrating neurocognitive changes early after cardiac surgery experience a return to baseline by three months.81
While subject to underrecognition on clinical grounds, the endpoint of stroke is at least in some regards more clearly objective than that of neurocognitive decline. Hopes that OPCAB would reduce the incidence of this endpoint have also been largely unmet. Multiple small studies have been reported with results on both sides of the argument. In two different meta-analyses, however, the incidence of stroke following OPCAB surgery was not statistically significantly reduced in comparison with ONCAB.82,83 Similarly, there has been no difference in stroke in most randomized trials.66–69,72,74 Arguments have been made that, as is the case for the issue of operative mortality, differences in stroke rate have not been demonstrable in these relatively small studies due to the low-risk nature of the populations studied. This notion is supported by the results of a recent analysis of 49 830 patients in the New York State Registry in which a lower incidence of stroke was demonstrable in OPCAB patients (adjusted OR 0.70, 95% confidence interval (CI) 0.57–0.86) (84). Unfortunately, most surgeons still place a clamp on the ascending aorta to perform proximal anastomoses for vein grafts when performing OPCAB. The operation is, therefore, not truly “no touch” with respect to the likely culprit source of cerebral embolic material, and it has been argued that the incidence of stroke after CABG may be determined more by the amount of aortic manipulation and less by the use of CPB.85
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


