CHAPTER 32 Surgery of the Diaphragm
A Deductive Approach
And on a day we meet to walk the line
And set the wall between us once again.
We keep the wall between us as we go….
He only says, “Good fences make good neighbors.”
EMBRYOLOGY
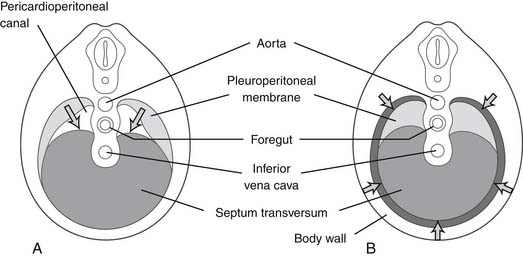
In utero, the diaphragm forms from the septum transversum and pleuroperitoneal folds.1 The septum transversum is an unpaired ventral membrane that separates the pericardium from the remainder of the thorax and creates the central trileaflet of the diaphragmatic tendon (Fig. 32-1).2 One of the trileaflet tendons lies within the right hemithorax, one within the left hemithorax, and the third beneath the pericardium. The dorsolateral portions of the diaphragm start with the formation of pleuroperitoneal folds. The mesothelium of the pleura binds to the mesothelium of the peritoneum in a membrane only two cell layers deep. Myotomes from C3, C4, and C5 then migrate from the lateral border toward the center of each hemithorax within the interspace between these two mesothelial layers in the 7th week of life. The outer rim of muscle of the diaphragm is from myotomes that carry nerve innervation from T7 through T12.2 The intestines return to the abdomen from the yolk sac in the 10th week of life and will be displaced into the chest if the diaphragm has not successfully formed (a congenital diaphragmatic hernia).
STRUCTURE AND FUNCTION
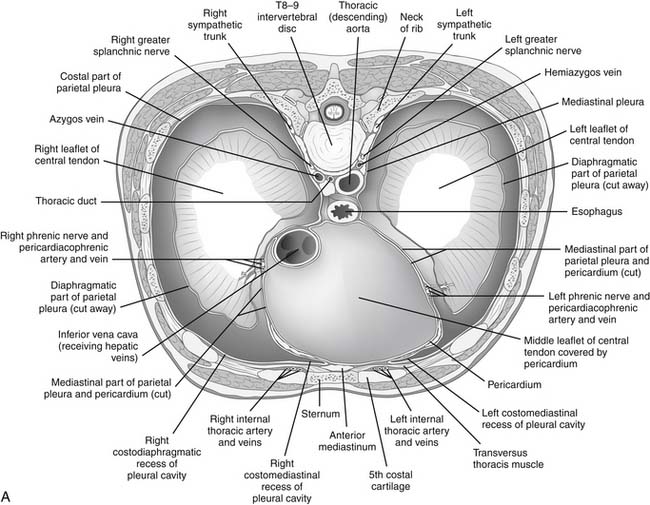
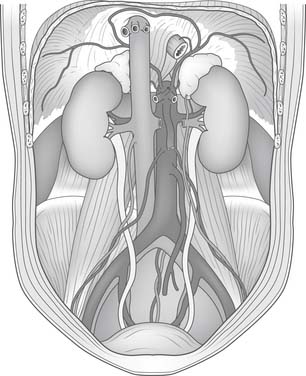
There are three natural openings within the diaphragm (Fig. 32-2). The aortic opening is the most posterior of the three and is formed from fibers composing the right and left diaphragmatic crura.3 This tunnel is actually behind the diaphragm, not within it, and contains the aorta, azygos vein, and thoracic duct. The esophageal hiatus is slightly more ventral from the aortic hiatus and consists of fibers passing between the aorta and the esophagus toward the right crus and fibers converging on the pericardial tendon. The opening of the inferior vena cava lies within the confluence of the tendons of the right hemithorax and the tendon beneath the pericardium.
The fan-shaped muscle of the diaphragm arises from the internal circumference of the thorax, with attachments to the sternum, the lower six or seven ribs, and the vertebral bodies of the lumbar vertebrae. Posteriorly, the muscle fibers originate from the aponeurotic arch of the ligamentum arcuatum externum, which overrides the psoas and quadratus lumborum muscle. Laterally, the fibers of the diaphragm interdigitate with slips from the transversalis muscle of the abdomen as they originate from the ribs.3 The right crus is larger and longer than the left and arises from the bodies of the upper three or four lumbar vertebrae. The left crus arises from the upper two lumbar vertebral bodies.
During inspiration, the first rib is elevated and fixed by the scalene muscles of the neck. The external intercostal muscles raise, in turn, each of the lower ribs. Raising these ribs, like a bucket handle that is attached to the sternum and vertebral column, enlarges the thorax and creates the negative pressure that ventilates the lung.3
The diaphragm is the major muscle of inspiration.4 In the resting state, the central tendon is displaced cephalad into the thorax by the positive intra-abdominal pressure. During contraction, the radial muscle fibers pull the tendon down toward the abdominal cavity like a drumhead. This further augments the negative intrathoracic pressure and further increases the positive pressure in the abdomen. The diaphragmatic crura contribute to the magnitude of displacement of the central tendon. In fact, if there were only circumferential attachments to the rib cage, the diaphragm would be limited in its ability to displace the lower ribs and to enlarge the thoracic cavity. The thicker fascicles of the crura, which lie at a 45- to 90-degree angle to the plane of the fan, pull on the anchoring lumbar spine like a lever and thus fix the central tendon in place. In descending, the fan of the diaphragm displaces the intra-abdominal viscera, which do not yield completely because they are bolstered by the anterior abdominal wall. The central tendon becomes a fixed point from which the radial muscles of the fan contract and are thus able to elevate the lower ribs. Even though the points of muscle attachment to the lumbar spine are more caudal than the attachments to the rib cage, the domed tendon acts as a fulcrum cephalad to the attachments to the rib cage. In fact, contraction of the diaphragm can raise the lower ribs only if the intra-abdominal viscera are in situ and not if the organs have been removed.3 Injuries to the crura have a more disproportionate effect on the respiratory function of the ipsilateral diaphragm than a similar injury to the peripheral muscle.
A forced inspiration descends the central tendon one or two rib interspaces. Under normal respiration, each hemidiaphragm provides between 15% and 25% of respiratory muscle function, with each side of the combined intercostals providing the remaining percentage.5,6 Under strained respiration, however, the diaphragm can increase its workload to provide up to 80% of the work of breathing.
PLEURAL AND PERITONEAL ATTACHMENTS
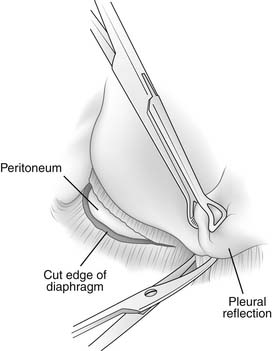
The pleura is tightly adherent to the top surface of the diaphragmatic central tendon and most of the musculature. It is impossible to separate the pleura from the central tendon of each hemidiaphragm. As the pleura curves off the chest wall and folds back on itself on the surface of the diaphragm, there is a circumferential diaphragmatic recess of approximately 1 cm that does not contain pleura.7 This recess of uncovered diaphragm is used to good advantage during an extrapleural dissection when the surgeon wraps his or her fingers into this recess and pulls downward, thus exposing the diaphragmatic musculature for division (Fig. 32-3).
ARTERIAL AND VENOUS ANATOMY
The superior phrenic arteries are located on the thoracic surface of the diaphragm. They are small branches from the lower thoracic aorta and traverse the posterior diaphragm over the top portion of each crus close to the mediastinum.3 They terminate in small anastomoses with the musculophrenic and pericardiophrenic arteries, which are both branches from the internal mammary artery. These two arteries also supply blood to the phrenic nerve and the pericardial fat pad.8
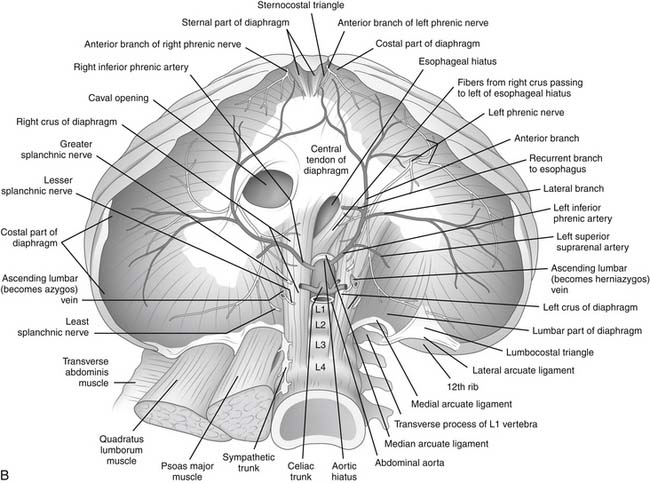
The inferior phrenic arteries lie on the undersurface of the crus and the dome of the diaphragm (Fig. 32-4). They are small paired vessels with frequent anatomic variations. They can originate separately or as a common trunk from the aorta above the celiac artery or from the celiac artery itself. Alternatively, a common trunk arising from either the aorta or the celiac artery gives rise to these two arteries. On occasion, one vessel originates from the aorta and the other emerges from one of the renal arteries. Diverging near the crura, the inferior phrenic arteries then course obliquely superior and lateral along the inferior surface of the diaphragm. The left inferior phrenic artery passes posterior to the esophagus and then runs anteriorly along the lateral side of the esophageal hiatus. The right inferior phrenic artery passes behind the inferior vena cava.3
Close to the posterior aspect of the central tendon, both the left and right inferior phrenic arteries divide into a medial and a lateral branch. The medial branch extends anteriorly, close to the mediastinum. Branches of this vessel traverse the muscular portion of the diaphragm to anastomose with the musculophrenic and pericardiophrenic arteries. The lateral branch of the inferior phrenic artery courses laterally and forms anastomoses with the lower intercostal arteries. The left inferior phrenic artery provides a minor contribution to the blood supply of the lower esophagus. Both the right and left inferior phrenic arteries have branches to the ipsilateral suprarenal gland. These branches are called the right and left superior suprarenal arteries.3
In general, the venous anatomy in this region parallels that of the arteries. The superior phrenic veins are small and drain anteriorly to the internal mammary vein. The much larger inferior phrenic veins parallel the course of the inferior phrenic arteries. The right vein empties directly into the inferior vena cava. The left vein usually has two branches, one of which drains into the left renal or suprarenal vein; the other passes anterior to the esophageal hiatus and empties into the inferior vena cava.3
LYMPHATICS
The lymphatics of the diaphragm drain toward the internal mammary chain anteriorly and the thoracic duct posteriorly. Smaller lateral lymphatic branches follow the course of the intercostal vessels along the lateral and posterior margins of the chest wall.3 Although these vessels are rarely seen in the nonpathologic state, engorged vessels are frequently seen on both the upper and lower surfaces of the diaphragm in patients who have congenital heart disease with elevated central venous pressures and in primary pathologic conditions of the lymphatics, such as cavernous lymphangioma.
DIAPHRAGMATIC INNERVATION
The phrenic nerve originates from the C3, C4, and C5 nerve roots and then enters the chest anterior to the subclavian artery. On the left side, the nerve lies medial to the internal mammary artery 64% of the time; on the right side, it lies medial to the internal mammary artery only 46% of the time.9 Thus, the left nerve is more susceptible to injury during mobilization of the left internal mammary artery through a median sternotomy incision.
The majority of diaphragmatic muscle originates from cervical myotomes innervated by fibers from the spinal nerve roots at cervical levels C3, C4, and C5. These fibers join and form the phrenic nerve, which elongates as the septum transversum migrates caudally. There is, however, an outer rim of diaphragmatic muscle that originates from migrating mesenchymal cells of the nearby body wall innervated by spinal nerves from thoracic levels T7-12. In addition, there is a contribution from mesenchyme associated with the foregut at levels L1-3 that coalesces to form the right and left crura.2,3
Despite the contributions from the thoracic and spinal nerve roots, the majority of the diaphragm is innervated by the phrenic nerve. Although the origin of the phrenic nerve and its proximal course through the mediastinum are well known, the distal extent of the nerve as it branches into the diaphragm proper is less well described. In 1956, Merendino and colleagues10 published the most descriptive and often-cited reference regarding this intradiaphragmatic portion of the phrenic nerve. Their anatomic findings and frequently adapted schematized drawings are based on electrical stimulation studies and gross dissection in dogs as well as on intraoperative dissection of approximately 40 human diaphragms.
The phrenic nerve usually divides at the level of the diaphragm or just above it. The right phrenic nerve enters the diaphragm just lateral to the inferior vena cava within the central tendon. The left phrenic nerve enters lateral to the left border of the heart just anterior to the central tendon within the muscle itself. The intradiaphragmatic course of the phrenic nerve can be predicted, even when it is not directly seen, by knowing the distribution of the four main motor divisions. The phrenic nerve first splits into an anterior and a posterior trunk (see Fig. 32-2B). The anterior trunk subsequently divides into a sternal and an anterolateral branch near the anteromedial border of the central tendon. The posterior trunk likewise divides into a crural and a posterolateral branch along the posteromedial border of the central tendon. The sternal and crural branches are short and continue to run in an anteromedial and posteromedial direction, respectively. The anterolateral and posterolateral branches are much longer and run close to the muscular fiber insertions into the central tendon. These two branches innervate the majority of the diaphragm. Their anatomic relation to one another is often described as a pair of handcuffs or manacles. These branches are often within the muscle layers and are not readily visible.
DIAPHRAGMATIC INCISIONS
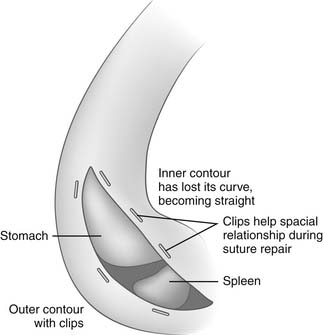
Diaphragmatic incisions can be divided into three groups: circumferential, central tendon, and radial. Circumferential incisions in the periphery result in little loss of function. These circumferential incisions, however, must be at least 5 cm lateral to the edge of the central tendon to avoid the posterolateral and anterolateral branches of the phrenic nerve. These incisions can be difficult to correctly realign after a long operation. Placement of surgical clips on each side of the muscular incision can greatly facilitate the correct spatial orientation on closing (Fig. 32-5).
TRAUMATIC DIAPHRAGMATIC INJURY
Blunt trauma that dramatically increases the intra-abdominal pressure may cause a central tendon rupture of the diaphragm. A common mechanism for rupture is seen in patients involved in high-speed motor collisions who are wearing seat belts. Imminent awareness of an approaching accident can cause inspiration with tensing of abdominal muscles and contraction of the diaphragm. This increases intra-abdominal pressure and in combination with additional force from the seat belt at impact can result in rupture of the diaphragm at the dome or central attachments. Diaphragmatic rupture occurs in 0.8% to 1.6% of patients arriving at the emergency department with blunt trauma.11 Because the liver protects the right hemidiaphragm from this mechanism of injury, most diaphragmatic ruptures from blunt trauma are recognized on the left side.
An abnormal diaphragm contour on plain chest radiograph or CT scan after a traumatic injury should raise the possibility of diaphragmatic injury. These findings may be easier to appreciate if a radiopaque nasogastric tube is in place at the time of imaging. Nonetheless, radiographic findings may still be as subtle as blurring of the costophrenic angle. Because of this subtlety, delayed diagnoses are not uncommon. Review of the literature suggests that the diagnosis is missed in as many as 66% in some series.12 This may be in part related to traditional CT imaging, which has yielded poor detection of diaphragmatic injury; however, reports of new helical CT scanners show increasing sensitivity as high as 84%.13 If clinical suspicion is high, minimally invasive techniques with laparoscopy or thoracoscopy have improved detection of occult diaphragmatic laceration, especially in penetrating left thoracoabdominal injuries.
DIAPHRAGMATIC ELEVATION
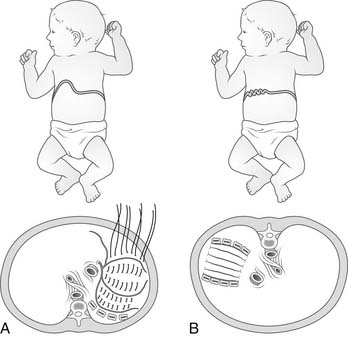
The two most common causes of diaphragmatic elevation are congenital eventration of the diaphragm and phrenic nerve palsy (Fig. 32-6A).
Congenital eventration of the diaphragm is a spectrum of disorders that share the underlying cause of impaired fetal myotome migration.14 Mild cases may lack only the central tendon, whereas severe cases may lack the central tendon as well as the entire muscular diaphragm. The area of congenitally missing muscle tissue is usually closed with a fused single membrane of pleura and peritoneum. This membrane is generally displaced within the ipsilateral hemithorax because of the absence of muscle.
Diaphragmatic elevation is poorly tolerated in neonates. The flaccid diaphragm itself compresses the lower lobe of the ipsilateral lung. In addition, if there is a large displacement of abdominal components into the negative-pressure thorax, this bulk mechanically shifts the mediastinum with compression of the contralateral lung. Thus, there is atelectasis of the ipsilateral lower lobe, compression of the left atrium and impediment to pulmonary venous blood flow, and contralateral lung compression with additional atelectasis.15 The end result may be complete pulmonary failure requiring intubation. Mechanical ventilation reinflates the atelectatic lungs and balances the mobile mediastinum. The ipsilateral diaphragm is shifted into the abdomen.
Surgical intervention is nearly mandatory in symptomatic infants with eventration; but in the adult population, the indications for surgery are less clear. In children, plication is readily successful in achieving weaning from ventilation. In adults, however, this is not the case. In addition, regarding nonventilated patients, the condition is better tolerated in adults, and in general only patients with progressive symptoms should be offered surgery. Causes of eventration in adults are usually related to phrenic nerve involvement through spinal cord disease, cervical trauma, infiltration of the nerve by tumors or lymph nodes, and iatrogenic injury. In certain conditions, conservative management will eventually lead to recovery of function; however, in the remaining patients, plication remains a low-risk procedure that continues to be underused. A study of patients who underwent unilateral plication by video-assisted thoracoscopic surgery (VATS) documented substantial increases in spirometry readings at 6 months after the procedure.16 The mechanism for improvement in these patients is due to increased tension of the diaphragmatic barrier between the thorax and abdomen. This allows patients to generate a more negative intrapleural pressure than is possible with a floppy, paralytic diaphragm.
A variety of techniques have been described in the literature for plication of the diaphragm. Severe forms of congenital eventration of the diaphragm with only a pleuroperitoneal membrane will require patching.14 A rim of rudimentary diaphragmatic tissue can generally be found around the lateral contour of the chest with enough substance to hold sutures to anchor the patch. Along the medial side, the patch can be stitched to the pericardium and the anterior thoracic spinal ligaments.
The easiest technique of plication is to place imbricating stitches within the central tendon of the diaphragm.17,18 If the sutures extend far enough from the edge of the diaphragmatic tendon, they can produce substantial caudal displacement of the tendon toward the abdominal cavity and allow expansion of the ipsilateral lower lobe as well as balancing of the mediastinum (Fig. 32-7).19 A number of variants of the central tendon repair exist and are performed by thoracoscopic, laparoscopic, or hybrid techniques. With improved videoscopic equipment and experience, the trend toward less invasive procedures will continue and may encourage more physicians to refer their patients for surgery. Mouroux and colleagues20 described a series of 12 patients who underwent thoracoscopically assisted plication with a utility 5-cm incision. A Duval grasper is used to invaginate the apex of the eventration caudad, creating a fold that is closed in two layers. A VATS reproduction of the traditional pleating or “accordion” repair was performed by Freeman and colleagues16 in 22 patients with use of an Endo Stitch (Ethicon Endo-Surgery, Cincinnati, OH) device to create six to eight parallel U stitches for patients with unilateral diaphragm paralysis. Improvement in pulmonary function and quality of life was seen along with a shortened hospital stay compared with patients who underwent thoracotomy. A total thoracoscopic technique with three ports has been described by Kim and associates21 and uses the additional adjuncts of carbon dioxide insufflation and steep reverse Trendelenburg position to push the diaphragm down and to increase the effective working space. Laparoscopic plication with four working trocars has been performed by Huttl and colleagues.22 Retention sutures are placed on the dome of the diaphragm and then used for traction. This allows creation of an intra-abdominal fold for plication with 12 to 15 U-type sutures. Finally, to counter the difficulty in placing adequate sutures through minimal access techniques, Moon and coworkers23 reported grasping and rolling of the redundant diaphragm followed by placement of noncutting linear endoscopic staplers underneath for creation of folds that are left in situ.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree