Supraventricular Tachycardias
Byron K. Lee, MD
Peter R. Kowey, MD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Heart rate greater than 100 bpm.
Heart rate greater than 100 bpm.
![]() Rhythm is supraventricular in origin.
Rhythm is supraventricular in origin.
 General Considerations
General Considerations
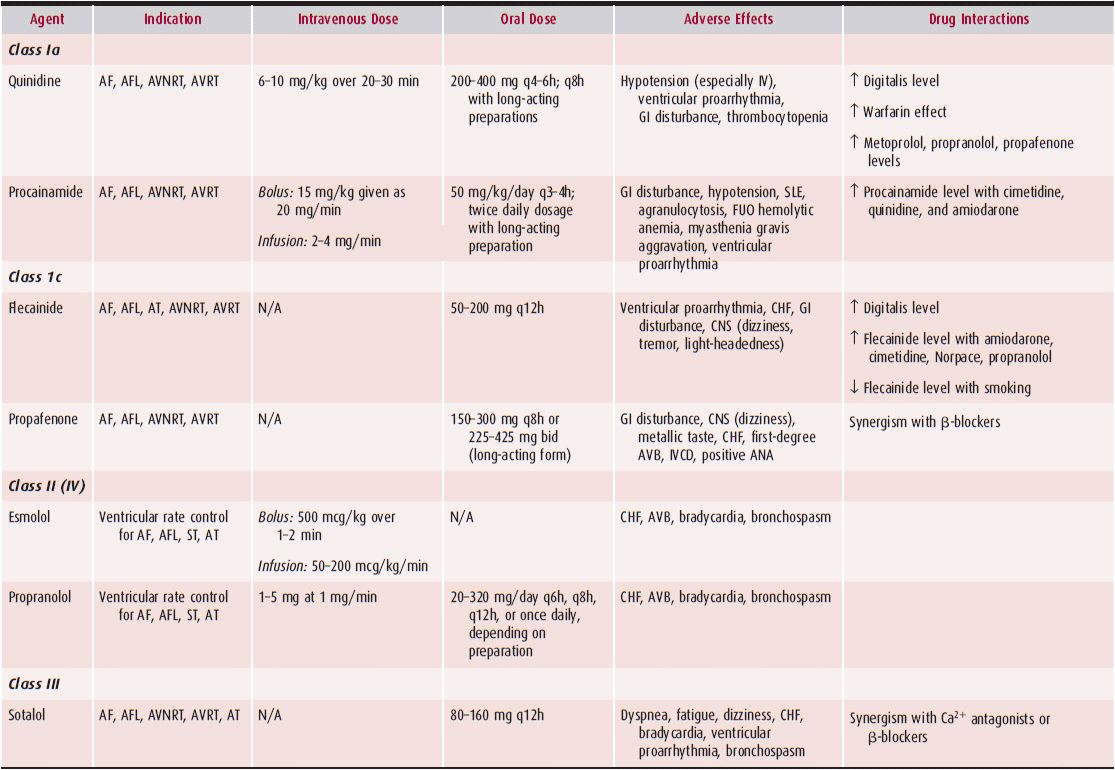
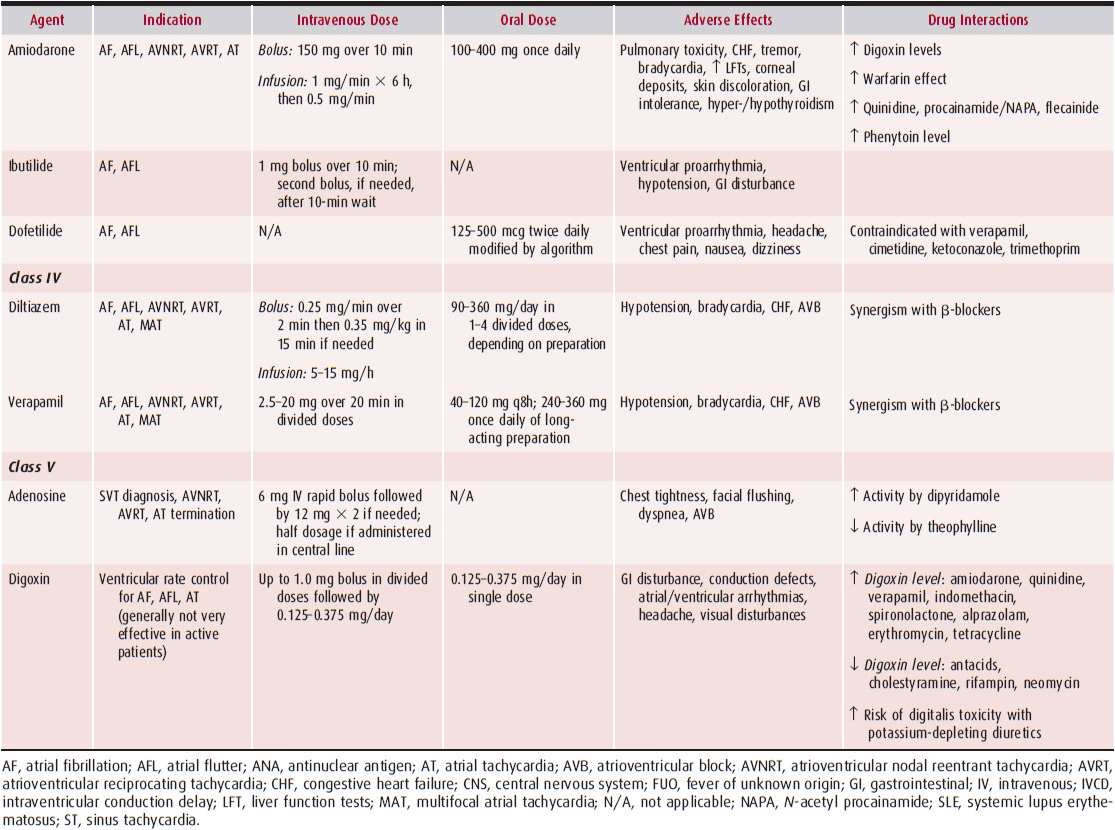
Supraventricular tachycardias (SVTs) are rapid rhythm disturbances originating from the atria or the atrioventricular (AV) node. In the absence of a bundle branch block, there is intact conduction to the ventricles via the right and left bundles leading to a narrow and normal-appearing QRS. Therefore, these arrhythmias are also often called narrow complex tachycardias. Since many of the SVTs are episodic, many clinicians also refer to this group of arrhythmias as paroxysmal SVTs. Radiofrequency ablation has become an important therapeutic option in the management of SVTs because of its ability to cure these arrhythmias safely. Table 11–1 outlines the pharmacologic therapy for SVTs.
Table 11–1. Antiarrhythmic Drugs for Supraventricular Tachycardias
 Pathophysiology & Etiology
Pathophysiology & Etiology
Arrhythmias occur as a result of three main mechanisms: reentry, which is most common; enhanced or abnormal automaticity; and triggered activity.
Reentrant arrhythmias sustain themselves by repetitively following a revolving pathway comprising two limbs, one that takes the impulse away from and one that carries it back to the site of origin. For reentry to exist, an area of slow conduction must occur, and each limb must have a different refractory period (see the discussion on AV nodal reentrant tachycardia). In this situation, pacing (by inducing refractoriness in one limb of the circuit) can typically initiate a reentrant tachycardia. Once established, pacing can also terminate the tachycardia by interfering with impulse propagation in one of the limbs.
The second mechanism, automaticity, refers to spontaneous and, often, repetitive firing from a single focus, which may either be ectopic or may originate in the sinus node. It should be noted that automaticity is an intrinsic property of all myocardial cells. This mechanism comprises two subcategories. Enhanced automaticity is defined as a focus that fires spontaneously and may originate in the sinus node, subsidiary pacemakers in the atrium including the Eustachian ridge, Bachmann bundle, coronary sinus and AV valves, the AV node, His-Purkinje system, and the ventricles. Abnormal automaticity is usually secondary to a disease process causing alterations in ionic flow that produces a lower (ie, more positive) resting diastolic membrane potential. Threshold potential is therefore more easily attained, thereby increasing the probability of a sustained arrhythmia.
The third mechanism, triggered arrhythmias, depends on oscillations in the membrane potential that closely follow an action potential. In the absence of a new external electrical stimulus, these oscillations, or after-depolarizations, cause new action potentials to develop. Thus, each new action potential results from the previous action potential. These arrhythmias can be produced by early or late after-depolarization, depending on the timing of the first after-depolarization relative to the preceding action potential (the one that spawned the triggered activity). In early after-depolarizations, membrane repolarization is incomplete, which allows an action potential to be initiated by a subthreshold stimulus. This type is often associated with electrolyte disturbance and may be the mechanism responsible for arrhythmogenesis related to the prolonged QT syndrome and torsades de pointes caused by quinidine. With delayed after-depolarization, membrane repolarization is complete, but an abnormal intracellular calcium load causes spontaneous depolarization. The reason for the high calcium levels is unclear, but it can be related to inhibition of the sodium pump by drugs such as digoxin. In either type of arrhythmia, the process may be repetitive and lead to a sustained tachycardia.
 General Diagnostic Approach
General Diagnostic Approach
A systematic approach to interpreting the 12-lead electrocardiogram (ECG) will allow accurate determination of the type of SVT in most cases (Figure 11–1). The first step is to determine whether the rhythm is regular or irregular. If it is irregular, the rhythm is likely either atrial fibrillation, atrial flutter with variable conduction, or multifocal atrial tachycardia (MAT). The appearance of the P waves or lack of P waves will usually distinguish between these three entities. In atrial fibrillation, there is chaotic atrial activity. In atrial flutter, P waves are seen at rate of 240–320 bpm. In MAT, there are P waves preceding each QRS complex, and there are at least three different P-wave morphologies.
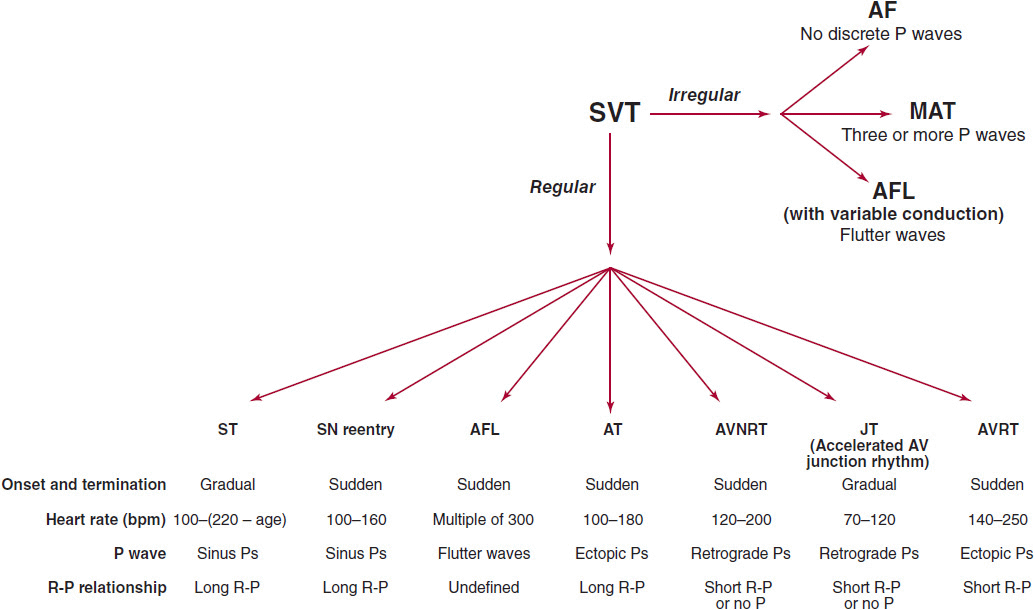
![]() Figure 11–1. Algorithm for distinguishing supraventricular tachycardias. AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reciprocating tachycardia; JT, junctional tachycardia; MAT, multifocal atrial tachycardia; SN, sinus node; ST, sinus tachycardia; SVT, supraventricular tachycardia.
Figure 11–1. Algorithm for distinguishing supraventricular tachycardias. AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reciprocating tachycardia; JT, junctional tachycardia; MAT, multifocal atrial tachycardia; SN, sinus node; ST, sinus tachycardia; SVT, supraventricular tachycardia.
If the SVT is regular, there are several main types of SVT to consider. The SVT could be sinus tachycardia, sinus node reentry, atrial flutter, atrial tachycardia, AV nodal reentrant tachycardia (AVNRT), junctional tachycardia, or atrioventricular reciprocating tachycardia (AVRT). The type of regular SVT can be usually identified by examining four aspects of the 12-lead ECG: onset and termination, heart rate, P wave morphology, and R-P relationship. Sinus tachycardia and junctional tachycardia typically have very gradual onset, whereas the other SVTs usually start and stop more suddenly. Rate can also be helpful since sinus tachycardia cannot typically go over 220-age bpm and the heart rate in atrial flutter is often a multiple of 300. P-wave morphology can be helpful since retrograde P waves (negative in the inferior leads: II, III, and aVF) favor AVNRT and junctional tachycardia. Finally, R-P relationship refers to the distance from the R wave to the next P wave during tachycardia. If this distance is longer than the P-R interval, the SVT is termed “long R-P,” whereas if this distance is short, it is termed “short R-P” (Figure 11–2).
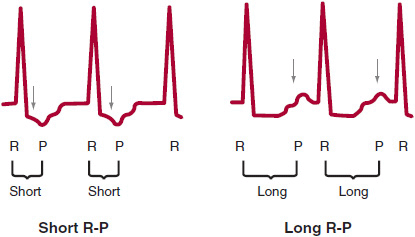
![]() Figure 11–2. Short R-P refers to a regular supraventricular tachycardia (SVT) where the R-P interval is shorter than the P-R interval. Long R-P refers to a regular SVT where the R-P interval is longer than the P-R interval.
Figure 11–2. Short R-P refers to a regular supraventricular tachycardia (SVT) where the R-P interval is shorter than the P-R interval. Long R-P refers to a regular SVT where the R-P interval is longer than the P-R interval.
SINUS TACHYCARDIA & SINUS NODE REENTRY
1. Sinus Tachycardia
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Onset and termination: gradual.
Onset and termination: gradual.
![]() Heart rate: 100 to (220 – age) bpm.
Heart rate: 100 to (220 – age) bpm.
![]() P wave: identical to normal sinus rhythm P wave.
P wave: identical to normal sinus rhythm P wave.
![]() R-P relationship: long.
R-P relationship: long.
 General Considerations
General Considerations
When the sinus node fires at a rate of more than 100 bpm, the rhythm is, with one exception, considered sinus tachycardia (see later section, Sinus Node Reentry). The onset and termination of sinus tachycardia are invariably gradual. The range for heart rate in sinus tachycardia is 100 to (220 – age) bpm; faster rates usually imply a different cause. Confirming that the tachycardic P waves are identical in morphology and axis to the normal sinus rhythm P waves is essential to the diagnosis. Like normal sinus rhythm, the R-P relationship in sinus tachycardia is typically long R-P, unless the patient has a very long P-R interval, which can usually be seen on the baseline ECG.
Sinus tachycardia is usually a physiologic response, activated when the body requires a higher heart rate to meet metabolic demands or maintain blood pressure. Common causes are exercise, hypotension, hypoxemia, heart failure, sepsis, fever, hyperthyroidism, fluid depletion, and blood loss.
The heart rate achieved is proportional to the intensity of the stimulus, but the rapidity with which the heart rate increases and decreases is a function of how quickly the stimulus is applied and withdrawn.
 Treatment
Treatment
Vagal maneuvers slow the tachycardia gradually but only while being performed; when the vagal stimulus is removed, the heart rate gradually returns to where it started.
Attempting to slow the heart rate pharmacologically can be detrimental because it counteracts the compensatory mechanism provided by the tachycardia. Therefore, management is usually focused on treating the underlying cause of the sinus tachycardia.
2. Sinus Node Reentry
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Onset and termination: sudden.
Onset and termination: sudden.
![]() Heart rate: 100–160 bpm.
Heart rate: 100–160 bpm.
![]() P wave: identical to normal sinus rhythm P wave
P wave: identical to normal sinus rhythm P wave
![]() R-P relationship: long.
R-P relationship: long.
 General Considerations
General Considerations
This uncommon rhythm accounts for less than 5% of SVTs. It uses the sinus node or perinodal tissue as a critical part of the reentrant circuit, producing P waves identical to those seen during normal sinus rhythm. The heart rate usually falls between 100 and 160 bpm. Like sinus tachycardia, the R-P relationship is typically long R-P. Unlike sinus tachycardia, sinus node reentry is initiated by an ectopic beat rather than a physiologic stimulus and possesses the characteristics typical of a reentrant circuit. It therefore begins and ends abruptly and responds to vagal maneuvers and pharmacologic interventions by terminating rather than slowing.
 Treatment
Treatment
The arrhythmia can be terminated quickly with intravenous adenosine, verapamil, or diltiazem, or via carotid massage. Long-term treatment uses β-blockers and calcium channel blockers. The largest reported series of patients treated with catheter ablation described success in all 10 patients. No complications were reported. Other smaller series found similar efficacy.
Sperry RE, et al. Radiofrequency catheter ablation of sinus node reentrant tachycardia. Pacing Clin Electrophysiol. 1993;16(11):2202–9. [PMID: 7505935]
ATRIAL FLUTTER
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Onset and termination: sudden.
Onset and termination: sudden.
![]() Heart rate: usually a multiple of 300.
Heart rate: usually a multiple of 300.
![]() P waves: flutter waves at 250–340 bpm.
P waves: flutter waves at 250–340 bpm.
![]() R-P relationship: undefined due to flutter waves.
R-P relationship: undefined due to flutter waves.
![]() Prominent neck vein pulsations of about 300/min.
Prominent neck vein pulsations of about 300/min.
 General Considerations
General Considerations
Atrial flutter is usually associated with organic heart disease and is second in frequency only to atrial fibrillation in post–coronary bypass surgery patients, with an incidence of up to 33%. With a typical atrial rate of 300 bpm (range 250–340 bpm), atrial flutter usually produces a “sawtooth” appearance (F waves). As is the case with atrial fibrillation (see Chapter 12), the ventricular rate depends on conduction through the AV node. Unlike atrial fibrillation, the ventricular impulses are transmitted at some integer fraction of the atrial rate. In rare circumstances, 1:1 conduction may occur. Fixed 2:1, 3:1, or 4:1 block is the usual scenario. However, variable block can also occur, leading to one of the three types of irregular SVTs (see Figure 11–1). If flutter is suspected but F waves are not clearly visible, vagal maneuvers or pharmacologic agents, such as adenosine, can help unmask the flutter waves by enhancing the degree of AV block.
 Pathophysiology
Pathophysiology
Atrial flutter occurs in a variety of forms; the most common is isthmus-dependent counterclockwise atrial flutter; followed by the isthmus-dependent clockwise atrial flutter; and then the atypical, nonisthmus-dependent variety. The counterclockwise flutter is recognized electrocardiographically by negative F waves in leads II, III, and aVF and positive F waves in lead V1. The single reentrant wavefront proceeds up the inter-atrial septum, across the roof of the right atrium, down the lateral wall, and across the inferior wall. Clockwise flutter, on the other hand, has positive F waves in leads II, III, and aVF and negative F waves in lead V1. The reentrant circuit in this case moves in the reverse direction. In both of these types of atrial flutter, the atrial rates range between 250 and 340 bpm.
 Clinical Findings
Clinical Findings
Symptoms attributable to atrial flutter are secondary to the ventricular response in addition to any underlying cardiac diseases. Dizziness, palpitations, angina-type chest pain, dyspnea, weakness, fatigue, and, occasionally, syncope may be the presenting symptoms. In those patients with poor left ventricular function, overt congestive heart failure may ensue.
Clinical evaluation is similar to that described for atrial fibrillation (see Chapter 12), but underlying heart disease is detected more often with atrial flutter than with fibrillation.
 Prevention
Prevention
Several antiarrhythmic agents can prevent recurrences of atrial flutter. It appears that both class Ia and Ic agents are effective. Class III agents, such as sotalol and amiodarone, can also work very well. Dofetilide, a newer class III agent, which blocks the rapid form of the delayed rectifier current, Ikr, has also been found effective in converting to and maintenance of sinus rhythm. Its administration requires initiation in the hospital and an ECG-monitored setting. Drugs that are contraindicated with its use include verapamil, ketoconazole, cimetidine, trimethoprim, prochlorperazine, megestrol, and hydrochlorothiazide. With regard to safety, dofetilide has an overall proarrhythmic event rate of approximately 0.9%, which is less than the 3.3% seen in patients with congestive heart failure or the 2.5% in patients with previous ventricular tachycardia.
It should be emphasized that an AV nodal blocking agent should be started before initiating a class I drug. If the AV node is unblocked, a type I agent could facilitate conduction of atrial flutter by improving nodal conduction or by slowing the flutter rate and paradoxically increasing the ventricular response.
 Treatment
Treatment
A. Conversion
Once the diagnosis of atrial flutter is made, assessment of the patient’s status will dictate whether to perform cardioversion immediately. Immediate cardioversion can be accomplished with synchronized direct-current (DC) cardioversion, rapid atrial pacing to interrupt the macroreentrant circuit, or intravenous infusion of an antiarrhythmic agent. For DC cardioversion, as little as 25 J may be all that is required; however, at least 50 J is recommended to avoid extra shocks, and 100 J will terminate almost all episodes of atrial flutter. The major drawback with DC cardioversion is the need to administer sedation.
Rapid atrial pacing is another method that may terminate the arrhythmia. Pacing is best performed in the right atrium at a rate faster than the flutter rate, which allows the circuit to be entered by the pacing impulse. If the extrinsic pacing rate exceeds the rate that can be sustained through the zone of slow conduction, the flutter wavefront can be interrupted and will no longer be present when the pacing is stopped. If the patient has a pacemaker or implantable cardiac defibrillator with an atrial lead, pace termination can be done painlessly via the device. An alternative method uses a swallowed transesophageal electrode. Because of the interposed tissue, a high current is often necessary to capture and pace the atrium reliably, which may cause significant discomfort to the patient. Of note, overdrive pacing may precipitate atrial fibrillation, which usually terminates spontaneously after several minutes. Should the atrial fibrillation persist, however, control of the ventricular response rate is typically easier when compared with atrial flutter.
Finally, rapid pharmacologic cardioversion can be considered with intravenous agents such as ibutilide. Ibutilide is a unique class III antiarrhythmic agent with a rate of conversion of approximately 60% in patients with atrial flutter of less than 45 days duration. Cardioversion can be expected within 30 minutes of administration. The major complication with this agent is the development of torsades de pointes, which can occur in up to 12.5% of patients, with 1.7% requiring cardioversion for sustained polymorphic ventricular tachycardia. These occur primarily within the first hour after administration. For this reason, patients given ibutilide are typically kept on monitor for several hours after administration. Procainamide is another intravenous agent that can be given to pharmacologically convert atrial flutter.
B. Rate Control
In general, controlling the ventricular rate in atrial flutter is more difficult than in atrial fibrillation. β-Blockers and calcium channel blockers are moderately effective in controlling the rate. Digoxin is less helpful since it only weakly blocks AV node conduction. Intravenous amiodarone, which has some β-blocking effect, has been shown to be at least as efficacious as digoxin.
C. Catheter Ablation and Other Modalities
The reentrant circuit in typical atrial flutter has been successfully mapped and includes an area of slow conduction called the isthmus, which is bound by the tricuspid annulus, the inferior vena cava, and the os of the coronary sinus. Ablation in the isthmus region interrupts the reentrant circuit and has been shown to be highly efficacious (90–100%) in permanently eliminating atrial flutter. In cost-effective analysis, ablation appears to be the preferred approach over cardioversion and pharmacologic prevention. Non–isthmus-dependent atypical atrial flutters can be more difficult to ablate. However, with current three-dimensional mapping systems (electro-anatomic and noncontact high resolution), even these types of atrial flutter are being ablated with high success rates.
If attempts to ablate atrial flutter fail, the ventricular rate can be controlled by transcatheter ablation of the AV node or His bundle. With a long-standing, there may be a subsequent improvement in left ventricular function.
D. Stroke Prophylaxis
Atrial flutter is a recognized cause of peripheral embolization and stroke. The current recommendation is to treat patients with atrial flutter just as atrial fibrillation in terms of stroke prophylaxis. Many patients have bouts of atrial fibrillation even after successful atrial flutter ablation. Therefore, rigorous monitoring after atrial flutter ablation is recommended to determine which patients need long-term aspirin or anticoagulant therapy.
Calkins H, et al. Results of catheter ablation of typical atrial flutter. Am J Cardiol. 2004;94(4):437–42. [PMID: 15325925]
Feld G, et al. Radiofrequency catheter ablation of type 1 atrial flutter using large-tip 8- or 10-mm electrode catheters and a high-output radiofrequency energy generator: results of a multicenter safety and efficacy study. J Am Coll Cardiol. 2004;43(8):1466–72. [PMID: 15093885]
Nakagawa H, et al. Characterization of reentrant circuit in macro-reentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow “focal” ablation. Circulation. 2001;103(5):699–709. [PMID: 11156882]
Tomson TT, et al. Risk of stroke and atrial fibrillation after radiofrequency catheter ablation of typical atrial flutter. Heart Rhythm. 2012;9(11):1779–84. [PMID: 22813577]
MULTIFOCAL ATRIAL TACHYCARDIA
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Heart rate: up to 150 bpm.
Heart rate: up to 150 bpm.
![]() P waves: three or more distinct P waves in a single lead.
P waves: three or more distinct P waves in a single lead.
![]() Variable P-P, P-R, and R-R intervals.
Variable P-P, P-R, and R-R intervals.
 General Considerations
General Considerations
Multifocal atrial tachycardia (MAT) is an irregular SVT that constitutes less than 1% of all arrhythmias. It is related to pulmonary disease in 60–85% of cases, with chronic obstructive pulmonary disease (COPD) exacerbation being the most common. In addition, MAT is precipitated by respiratory failure, acute decompensated cardiac function, and infection. It has also been reported to be associated with hypokalemia, hypomagnesemia, hyponatremia, pulmonary embolism, cancer, and valvular heart disease, and can also occur in the postoperative setting. It affects children and adults. Distention of the right atrium from elevated pulmonary pressures causes multiple ectopic foci to fire, with ventricular rates not usually exceeding 150 bpm. Whether this rhythm is due to abnormal automaticity or triggered activity is uncertain, but the ability of verapamil to suppress the ectopic atrial activity by virtue of its calcium channel–blocking properties supports the latter assumption.
Three ECG criteria must be met to diagnose MAT (Figure 11–3): (1) the presence of at least three distinct P-wave morphologies recorded in the same lead; (2) the absence of one dominant atrial pacemaker; and (3) varying P-P, P-R, and R-R intervals.
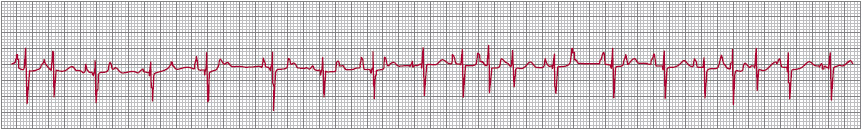
![]() Figure 11–3. Multifocal atrial tachycardia. The presence of at least three distinct P-wave morphologies, the absence of one dominant pacemaker focus, and varying P-P, R-R, and PR intervals establish the diagnosis.
Figure 11–3. Multifocal atrial tachycardia. The presence of at least three distinct P-wave morphologies, the absence of one dominant pacemaker focus, and varying P-P, R-R, and PR intervals establish the diagnosis.
MAT is often misdiagnosed as atrial fibrillation. Although both are irregular, the former has distinct P waves with an intervening isoelectric baseline. At times, MAT may progress to atrial fibrillation.
 Treatment
Treatment
The primary treatment for MAT should be directed at the underlying disease state. Oral and intravenous vera-pamil and several formulations of intravenous β-blockers have been effective to varying degrees in either slowing the heart rate (without terminating the rhythm) or in converting the arrhythmia to sinus rhythm. Intravenous magnesium and potassium, even in patients with serum levels of these electrolytes within the normal range, convert a significant percentage of these patients to sinus rhythm. Digoxin is not effective in treating this condition. Moreover, treatment with digoxin may precipitate digitalis intoxication. In addition, if the arrhythmia is secondary to delayed after-depolarizations, further aggravation may occur with digitalis because this drug increases delayed after-depolarizations. Medications that cause atrial irritability, such as theophylline and β-agonists, should be withdrawn whenever possible.
Application of radiofrequency energy for both AV node modification and AV node ablation with subsequent implantation of a pacemaker has been reported. The numbers of patients in the studies were very small, and there are no long-term results. Nevertheless, ablation of the AV node has been shown to reduce symptomatic MAT, resulting in improved quality of life, reduced hospital admissions for recurrent symptomatic MAT, and improved left ventricular function.
 Prognosis
Prognosis
Because of the severity of the precipitating underlying diseases, MAT portends a poor outcome. Mortality during the hospitalization when the arrhythmia is first diagnosed is between 30% and 60%, with death being attributed to the disease state rather than the tachycardia itself. In one study of patients with pulmonary disease who were admitted for acute respiratory failure, the in-hospital mortality rate for those with MAT was 87%, compared with 24% for those in a different rhythm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


