Fig. 28.2 Coronary care unit (CCU) monitoring showing bradycardia, rhythm uncertain (possibly sinus with a long PR interval, possibly heart block), then a very fast and disorganized ventricular arrhythmia, losing amplitude quickly and degenerating into ventricular fibrillation.
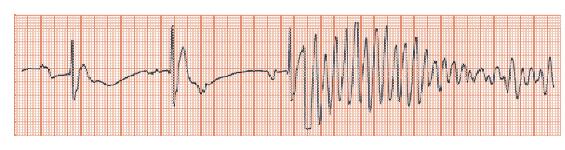
Fig. 28.3 Broad complex tachycardia. P waves cannot be seen. The ventricular complexes are of one shape (monomorphic), broad with right axis deviation. The V1 complex is positive (i.e. shows a positive not negative deflection), with the initial deflection being the most positive (quite unlike right bundle branch block, where there is a late large positive deflection). These appearances are virtually pathognomonic of ventricular tachycardia
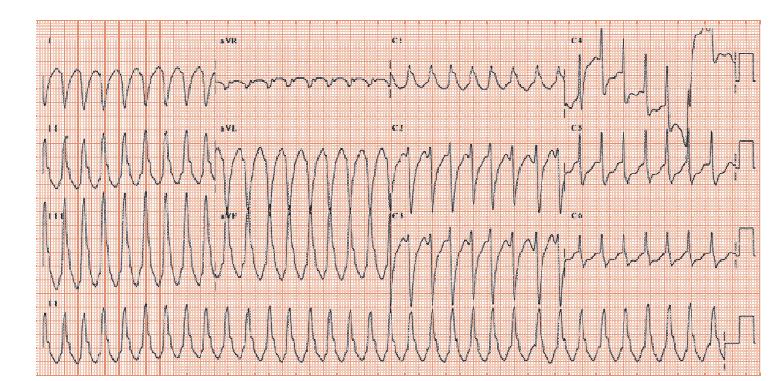
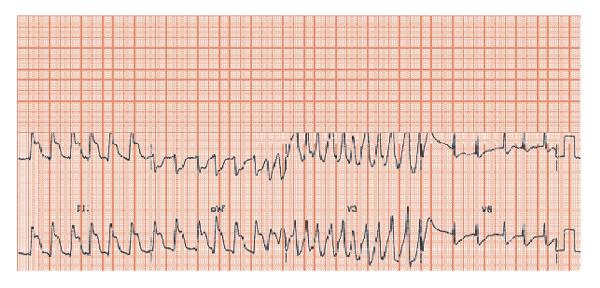
Fig. 28.4 Initial rhythm uncertain, possibly atrial fibrillation (fast slightly irregular RR intervals). Massive ST elevation inferiorly (leads II, III, aVF) indicative of inferior ST segment elevation myocardial infarction (STEMI). When the ECG recording of leads V1–3 was made, a fast, moderately organized arrhythmia occurred – this is a burst of ischaemically mediated ventricular tachycardia.
Sudden cardiac death (SCD) arises from a sudden loss of heart function, resulting in death within 1h. It accounts for 50% of cardiac deaths.
Mechanisms and management of SCD
Most SCD relates to ventricular arrhythmias. A few cases relate to primary bradyarrhythmias (Fig. 28.1). Most ventricular tachycardia/ventricular fibrillation (VT/VF) relates to coronary disease, 25% to heart failure/cardiomyopathy and 5% to aortic stenosis. Other rarer non-arrhythmic causes of SCD include pump failure from large myocardial infarctions (MIs), pulmonary embolisms and exsanguination or pericardial tamponade from aortic dissection. Some non-cardiac conditions cause sudden death, but by definition not sudden cardiac death, e.g. large strokes, brisk gastrointestinal bleeds, etc.
All arrhythmias associated with a loss of cardiac output clearly require immediate treatment. A few can be resuscitated: those due to bradyarrhythmias require pacing, those with tachyarrhythmias require determination of the underlying cardiac disease and requirement for specific treatment, and often also anti-tachycardic device therapy.
Cardiac conditions underlying SCD
- Ischaemic heart disease (IHD) without previous infarction underlies SCD either when new infarction/ischaemia provokes VT/VF (more likely in those with left ventricular hypertropy [LVH]) or causes the loss of so much myocardium that cardiac output cannot be sustained.
- Ishaemic heart disease with remote MI:
- The MI-related scar provides a substrate for a re-entry circuit allowing sustained monomorphic VT, which degenerates into VF. Such a scar delays normal depolarization and marginally prolongs the QRS complex. This can be seen as a late positive deflection on a highly amplified averaged QRS complex (a ‘late potential’ from a signal averaged ECG [SAECG], where 100–1000 beats are averaged to improve the signal to noise ratio). Most patients with monomorphic VT have late potentials; most patients with late potentials do not have VT. Factors in addition to late potentials are needed for VT, including new ischaemia, hypokalaemia (possibly through QT interval lengthening), and abnormal autonomic function, amongst others.
- The chance of VT/VF relates inversely to LV function.
- The MI-related scar provides a substrate for a re-entry circuit allowing sustained monomorphic VT, which degenerates into VF. Such a scar delays normal depolarization and marginally prolongs the QRS complex. This can be seen as a late positive deflection on a highly amplified averaged QRS complex (a ‘late potential’ from a signal averaged ECG [SAECG], where 100–1000 beats are averaged to improve the signal to noise ratio). Most patients with monomorphic VT have late potentials; most patients with late potentials do not have VT. Factors in addition to late potentials are needed for VT, including new ischaemia, hypokalaemia (possibly through QT interval lengthening), and abnormal autonomic function, amongst others.
- Left ventricular dysfunction from any non-IHD cause is a powerful substrate for VT/VF, though the risk is less than for those with post-MI LV dysfunction.
- Aortic stenosis.
- Cardiomyopathy (hypertrophic, right ventricular and other rare ones).
- Primary electrical abnormalities, including long QT syndromes, Brugada syndrome and Wolff–Parkinson–White syndrome.
- Pro-arrhythmic drugs are a common and under-recognized cause of ventricular arrhythmias, especially in acquired long QT syndrome.
ECG risk-markers for SCD
The greatest individual risk is in those with impaired LV function and:
- Prolonged QRS duration, the broader the higher the risk.
- Prolonged QT interval, if marked, is associated with an arrhythmic tendency.
- Frequent ventricular arrhythmias; VPC rate correlate weakly with SCD post-MI; non-sustained VT correlates more strongly.
- ‘Late-potentials’, a necessary but not sufficient condition in post-MI related SCD.
- Autonomic imbalance, as measured by low 24-h heart rate variability (see Chapter 63), identifies an ‘at-risk’ population.
- Electrophysiology studies: in those with impaired LV function, ambient non-sustained VT and inducible VT not suppressible with drugs, the subsequent risk of SCD is high.
Long QT syndromes
Long QT syndromes underlie SCD due to torsade-de-pointes (TDP) ventricular tachycardia. The QT interval reflects action potential duration (APD), and when APD is prolonged, QT interval lengthens. Pathological APD prolongation promotes afterdepolarizations (see Chapter 38) that provoke or underlie sustained ventricular arrhythmias (especially TDP). Afterdepolarizations are commonest when the QT interval is longest, i.e. low heart rate or at night and are promoted by adrenergic switch-on. There are two forms of long QT syndrome:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


