The prevention of stroke and other thromboembolic events plays a crucial role in the management of patients with atrial fibrillation. Not all patients with atrial fibrillation are equal in terms of thromboembolic risk; therefore, not all will benefit from oral anticoagulation treatment. The general principle is that the expected benefit of anticoagulation in reduction of thromboembolic risk must exceed the expected harm caused by possible bleeding. Some guidelines have focused on a categorical approach to stroke prevention, with a focus on identifying patients at high risk for oral anticoagulation. Various current guidelines recommend assessment of stroke risk using the CHADS 2 or CHA 2 DS 2 -VASc scores to initially detect patients at low risk who require no antithrombotic therapy. However, the scores do not incorporate all possible risk factors causing a high thromboembolic risk. Factors such as impaired renal function, obstructive sleep apnea, and echocardiographic and biochemical or coagulation parameters can also predict adverse thromboembolic events. The present review aims to describe biomarkers whether blood, urine, imaging (cardiac or cerebral), or clinical that go beyond the CHA 2 DS 2 -VASc score and potentially aid stroke risk assessment. Although useful in some cases, the presented parameters should be perhaps used to further refine initial identification of patients at low risk, after which effective stroke prevention can be offered to those with ≥1 additional stroke risk factors.
Atrial fibrillation (AF) is an independent risk factor for stroke, but not all patients with AF have equal stroke risk, and therefore, not all should be considered in the same manner. Age, gender, and co-morbidities influence thromboembolic risk, and it would be simplistic to regard that all risk factors carry equal weight. Various stroke risk factors had led to a development of several risk stratification scores, to aid clinical decision making. The general principle is that the expected benefit in reduction of ischemic stroke risk associated with anticoagulation must exceed the expected bleeding-related harm. Many of the current guidelines of many scientific societies enforce making the decision on whether to use anticoagulation therapy on one of the 2 most widely used risk-stratification schemes, that is, the CHADS 2 or CHA 2 DS 2 -VASc scores. However, these scores were designed to be simple and practical, and thus, include the common stroke risk factors seen in everyday clinical practice. These scores do not include many of the less common stroke risk factors, imaging or biomarkers, and in this review article, we aim to look beyond the common stroke risk factors within these 2 clinical risk scores.
CHADS 2 and CHA 2 DS 2 -VASc Scores: Where Are We Now?
Over the last few decades, several epidemiologic studies aimed to describe factors influencing the stroke risk in patients with AF. The CHADS 2 score was developed from the risk factors for stroke seen in the nonwarfarin arms of the historical trials cohorts, amalgamating the risk stratification schemes of the AF Investigators and the Stroke Prevention in Atrial Fibrillation Investigators. The CHADS 2 score was validated in the hospitalized cohort of the National Registry of Atrial Fibrillation study. Using the CHADS 2 score, points are assigned for history of (recent decompensated) congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and history of stroke or transient ischemic attack. The CHADS 2 score was simple and promptly found its place in clinical practice. Over the years after its introduction, the limitations of the CHADS 2 score were increasingly evident. Indeed, external validations of the score and reviews that compared CHADS 2 with other risk assessment schemes confirmed the poor ability of the CHADS 2 score in distinguishing patients at “high risk” who will benefit from anticoagulation. Also, the CHADS 2 score poorly identified patients at low risk, resulting in high rates of thromboembolic events even in patients who are presumably at low risk.
In 2010, the CHA 2 DS 2 -VASc was proposed, which incorporated factors omitted in the original CHADS 2 score but included additional “non-CHADS 2 risk factors,” namely age 65 to 74 years, vascular disease (myocardial infarction, peripheral artery disease, and complex aortic plaque), and female gender ( Table 1 ). Many subsequent analyses showed that the CHA 2 DS 2 -VASc particularly helped distinguish patients who were at low risk in whom antithrombotic therapy was not indicated. Table 2 summarizes the annual stroke and thromboembolism risk according to the CHADS 2 and CHA 2 DS 2 -VASc scores.
| CHADS 2 | Letter | CHA 2 DS 2 -VASc | ||
|---|---|---|---|---|
| Description ∗ | Points | Points | Description ∗ | |
| Recent heart failure exacerbation | 1 | C | 1 | Moderate to severe systolic left ventricular dysfunction, LVEF ≤40%, or recent decompensated heart failure requiring hospitalization |
| History of hypertension | 1 | H | 1 | History of hypertension |
| Age ≥75 years | 1 | A | 2 | Age ≥75 years |
| Diabetes mellitus | 1 | D | 1 | Diabetes mellitus |
| History of stroke or transient ischemic attack | 2 | S | 2 | History of stroke, transient ischemic attack or thromboembolism |
| V | 1 | History of myocardial infarction, complex aortic plaque or peripheral artery disease | ||
| A | 1 | Age 65 to 74 years | ||
| Sc | 1 | Female sex | ||
| CHADS 2 | Points | CHA 2 DS 2 -VASc |
|---|---|---|
| Annual risk | Annual risk | |
| 1.7% (1.5-1.9) | 0 | 0.8% (0.6-1.0) |
| 4.8% (4.5-5.1) | 1 | 2.0% (1.7-2.4) |
| 7.3% (6.9-7.8) | 2 | 3.7% (3.4-4.1) |
| 15.5% (14.6-16.4) | 3 | 5.9% (5.5-6.3) |
| 21.6% (20.0-23.2) | 4 | 9.3% (8.7-9.9) |
| 19.7% (16.9-23.0) | 5 | 15.3% (14.4-16.2) |
| 22.4% (14.6-34.3) | 6 | 19.7% (18.2-21.4) |
| 7 | 21.5% (18.8-24.6) | |
| 8 | 22.3% (16.3-30.8) | |
| 9 | 23.6% (10.6-52.6) |
Given that risk scores solely based on clinical risk factors only have modest predictive value for patients at high risk and that previous guideline strategies focused on a categorical (i.e., low, moderate, and high) risk strata approach to stroke prevention led to undertreatment of the patients at high risk, a different focus was clearly needed. In addition, stroke risk is a continuum, and the artificial categorization of stroke risk into low, moderate, and high risk strata simply had not improved optimal thromboprophylaxis in patients with AF, especially in the “high-risk” category.
The approach associated with the introduction of CHA 2 DS 2 -VASc score changed clinical practice. In the 2012 focused update of the European Society of Cardiology guidelines, there was a recommendation that instead of focusing on the identification of patients at high risk, a clinical practice shift was recommended so that the initial step was the identification of patients who are “truly at low risk” (i.e., CHA 2 DS 2 -VASc score 0 in males and 1 in females) who did not need any antithrombotic therapy. The subsequent step was to offer effective stroke prevention (i.e., oral anticoagulation) to patients with one or more additional stroke risk factors. Indeed, Olesen et al found that a CHADS 2 = 0 was not “low risk” and that the annual stroke risk in this group may be as high as 3.2%.
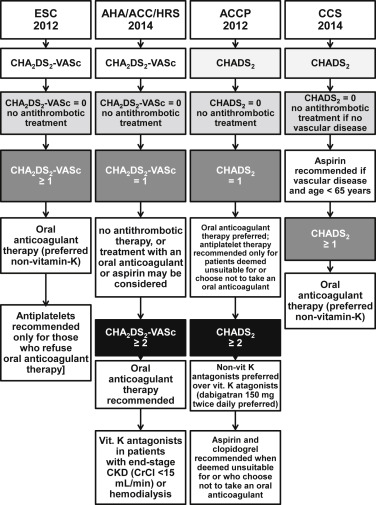
Guidelines do differ in their approaches. In the European Society of Cardiology and American Heart Association/American College of Cardiology/Heart Rhythm Society, the use of CHA 2 DS 2 -VASc score is recommended. However, in the European Society of Cardiology guidelines, only patients with CHA 2 DS 2 -VASc score = 0 in males (or 1 in females) are not recommended oral anticoagulation, whereas all the others with ≥1 stroke risk factors are recommended (or should consider) oral anticoagulants. The 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society agrees with no anticoagulation in patients with CHA 2 DS 2 -VASc score = 0, but in those with a CHA 2 DS 2 -VASc score = 1, the recommendation is “nothing, aspirin, or oral anticoagulation.”
The guidelines of the Canadian Cardiovascular Society and the American College of Chest Physicians also opt for using the CHADS 2 score. Again, they also agree on no coagulation in patients with CHADS 2 score = 0, but the Canadian Cardiovascular Society assigns oral anticoagulation to all patients with a score ≥1 or those aged ≥65 years, with aspirin recommended in patients with AF aged <65 years with vascular disease. The 2012 American College of Chest Physicians guidelines are based on the CHADS 2 score, but in those with a CHADS 2 score = 0, they recommend consideration of “non-CHADS 2 ” risk factors (i.e., age 65 to 74, vascular disease, and female gender) where oral anticoagulation may be considered. Details regarding the differences in all 4 guidelines are described in Figure 1 .
Beyond the CHA 2 DS 2 -VASc Score
As discussed previously, the CHA 2 DS 2 -VASc score includes the common stroke risk factors seen in everyday clinical practice. Apart from left ventricular impairment or complex aortic plaque on echocardiography, the CHA 2 DS 2 -VASc score includes neither any imaging or biomarker parameters nor some of the less common clinical stroke risk factors.
Impaired renal function
Chronic kidney disease (CKD) is common in patients with AF, and between 10% and 15% of all patients with AF meet the criteria for the diagnosis. The prevalence of AF also increases with the severity of renal function impairment. For example, in patients with AF with estimated glomerular filtration rate <45 ml/min, the prevalence of AF was approximately 20%. Common risk factors are responsible for development of both AF and CKD–indeed, these include hypertension, diabetes mellitus, impaired endothelial function, and inflammation–and all are associated with elevated thromboembolic risk.
Impaired renal function impairment is associated with increased thromboembolic risk in AF. For example, in female patients with AF, a history of stroke or transient ischemic attack has been linked with sevenfold increase in the stroke risk, whereas renal dysfunction resulted in an 11-fold increase.
One analysis of AF patients categorized according to the risk assessed in the CHADS 2 score found that in the control group (patients with normal renal function), approximately 70% of patients were classified as CHADS 2 0 or 1 points, whereas in the group with CKD stage >III, almost 70% had CHADS 2 ≥2 points. This accentuated risk with CKD in patients with AF led to a proposal to include CKD into the existing CHA 2 DS 2 -VASc scheme, whereby the little “c” on the end of the acronym was proposed to be translated as “chronic severe renal impairment.”
In the interim, the R 2 CHADS 2 score was proposed based on an analysis of the ROCKET (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) AF study. The initial “R 2 ” in the acronym stood for renal dysfunction and meant that a patient was assigned 2 additional points when his glomerular filtration rate was <60 ml/min. The initial analysis showed that the predictive value of this score was comparable with CHADS 2 and CHA 2 DS 2 -VASc, but has several major limitations. Also, the derivation of R 2 CHADS 2 was from a selected anticoagulated clinical trial cohort, which only included subjects at high risk (CHADS 2 score ≥2 and those with score = 2 were capped at 10%), and those with creatinine clearance <30 ml/min were excluded.
Various “real-world” cohorts with a broad spectrum of stroke risk and renal function showed that renal function did not significantly improve the prediction value of the CHA 2 DS 2 -VASc score for identifying patients at high risk. For example, Roldán et al showed that adding CKD to CHADS 2 or CHA 2 DS 2 -VASc in patients with AF did not provide additional benefit in risk stratification. Renal impairment is associated with heart failure, age, diabetes, vascular disease, and so forth, which are components within the CHA 2 DS 2 -VASc score. Thus, it would be difficult to show an incremental predictive advantage in stroke prediction by adding renal impairment to CHA 2 DS 2 -VASc.
There is little doubt that CKD in AF patients represents a high-risk group. For example, Lin et al identified 338 subjects with CHA 2 DS 2 -VASc score of 0 to 1 point and followed them for systemic thromboembolic events, including acute ischemic stroke, transient ischemic attack, and peripheral artery embolism. In patients at low risk without the CKD, annual event rate was 0.2%, whereas with CKD patients, the event rate was 2.9% (p <0.001). Similar conclusions were evident in a study of patients after AF ablation, where in patients with CHA 2 DS 2 -VASc score of 0 to 1, the presence of CKD was associated with a 14-fold increase in the rate of thromboembolic events. Therefore, all patients with AF with CKD are at increased risk of thromboembolic events.
Obstructive sleep apnea
Obstructive sleep apnea is among the more novel cardiovascular risk factors, associated with a number of cardiovascular diseases, including hypertension, coronary artery disease, myocardial infarction, stroke, or arrhythmias. This translates into elevated risk of stroke seen in, but this seems to be related to several factors, including a prothrombotic state, endothelial dysfunction, intermittent hypoxia, variations in intrathoracic pressure, and recurrent arousals associated with increased heart rate and blood pressure.
The prevalence of obstructive sleep apnea in patients with AF is very high. In the general population, the estimated obstructive sleep apnea prevalence is 2% to 4%, whereas in patients with AF referred for ablation, obstructive sleep apnea was found in nearly 46% of cases. Recently, obstructive sleep apnea and AF, along with erectile dysfunction, have been proposed to be a single clinical entity.
Obstructive sleep apnea (OSA) is directly and independently associated with elevated thromboembolic risk in AF. Our study included 254 patients with AF who underwent polygraphy for a diagnosis of OSA and had their thromboembolic risk assessed using the CHADS 2 and CHA 2 DS 2 -VASc scores. The patients with obstructive sleep apnea had significantly greater CHADS 2 and CHA 2 DS 2 -VASc scores and along with the increasing obstructive sleep apnea severity expressed by greater values of the apnea-hypopnea index, also the mean risk score increased. A study by Yaranov et al found that in patients with CHA 2 DS 2 -VASc score = 0, the presence of obstructive sleep apnea increased the risk of stroke by 62%.
Other clinical risk factors associated with increased thromboembolism
Amyloid is a fibrous protein aggregate composed of various forms of inappropriately folded proteins and polypeptides. It is associated with degenerative diseases of central nervous system and the heart. One of its subforms, serum amyloid protein A, is considered to be an inflammatory cytokine. Elevated serum amyloid protein A levels have been found in patients with AF and are associated with atrial remodeling and inflammatory state and promote vascular thrombosis. This association is confirmed by higher levels of serum amyloid protein A found in patients with venous thromboembolism compared with control group. A different type of the protein, namely β amyloid, is extensively deposited within the walls of small vessels, especially in the elderly. The presence of amyloid angiopathy in the cerebral vessels predisposes not only to greater risk for thromboembolic events but also for intracranial hemorrhage related to anticoagulation.
Also, some myocardial diseases such as obstructive hypertrophic cardiomyopathy (HC) are linked with alterations in coagulation parameters. In patients with obstructive HC, various indicators of the coagulation activation are found. They include elevated levels of plasma fibrinopeptide A and thrombin-antithrombin HI complex. Nearly 15% of patients with HC develop AF, and such patients are at high risk of stroke and thromboembolism. Others have proposed the addition of hyperlipidemia and smoking to CHA 2 DS 2 -VASc, the so-called CHA 2 DS 2 -VASc-HS score, proposed to predict vascular events, not thromboembolism.
Beyond the CHA 2 DS 2 -VASc Score
As discussed previously, the CHA 2 DS 2 -VASc score includes the common stroke risk factors seen in everyday clinical practice. Apart from left ventricular impairment or complex aortic plaque on echocardiography, the CHA 2 DS 2 -VASc score includes neither any imaging or biomarker parameters nor some of the less common clinical stroke risk factors.
Impaired renal function
Chronic kidney disease (CKD) is common in patients with AF, and between 10% and 15% of all patients with AF meet the criteria for the diagnosis. The prevalence of AF also increases with the severity of renal function impairment. For example, in patients with AF with estimated glomerular filtration rate <45 ml/min, the prevalence of AF was approximately 20%. Common risk factors are responsible for development of both AF and CKD–indeed, these include hypertension, diabetes mellitus, impaired endothelial function, and inflammation–and all are associated with elevated thromboembolic risk.
Impaired renal function impairment is associated with increased thromboembolic risk in AF. For example, in female patients with AF, a history of stroke or transient ischemic attack has been linked with sevenfold increase in the stroke risk, whereas renal dysfunction resulted in an 11-fold increase.
One analysis of AF patients categorized according to the risk assessed in the CHADS 2 score found that in the control group (patients with normal renal function), approximately 70% of patients were classified as CHADS 2 0 or 1 points, whereas in the group with CKD stage >III, almost 70% had CHADS 2 ≥2 points. This accentuated risk with CKD in patients with AF led to a proposal to include CKD into the existing CHA 2 DS 2 -VASc scheme, whereby the little “c” on the end of the acronym was proposed to be translated as “chronic severe renal impairment.”
In the interim, the R 2 CHADS 2 score was proposed based on an analysis of the ROCKET (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) AF study. The initial “R 2 ” in the acronym stood for renal dysfunction and meant that a patient was assigned 2 additional points when his glomerular filtration rate was <60 ml/min. The initial analysis showed that the predictive value of this score was comparable with CHADS 2 and CHA 2 DS 2 -VASc, but has several major limitations. Also, the derivation of R 2 CHADS 2 was from a selected anticoagulated clinical trial cohort, which only included subjects at high risk (CHADS 2 score ≥2 and those with score = 2 were capped at 10%), and those with creatinine clearance <30 ml/min were excluded.
Various “real-world” cohorts with a broad spectrum of stroke risk and renal function showed that renal function did not significantly improve the prediction value of the CHA 2 DS 2 -VASc score for identifying patients at high risk. For example, Roldán et al showed that adding CKD to CHADS 2 or CHA 2 DS 2 -VASc in patients with AF did not provide additional benefit in risk stratification. Renal impairment is associated with heart failure, age, diabetes, vascular disease, and so forth, which are components within the CHA 2 DS 2 -VASc score. Thus, it would be difficult to show an incremental predictive advantage in stroke prediction by adding renal impairment to CHA 2 DS 2 -VASc.
There is little doubt that CKD in AF patients represents a high-risk group. For example, Lin et al identified 338 subjects with CHA 2 DS 2 -VASc score of 0 to 1 point and followed them for systemic thromboembolic events, including acute ischemic stroke, transient ischemic attack, and peripheral artery embolism. In patients at low risk without the CKD, annual event rate was 0.2%, whereas with CKD patients, the event rate was 2.9% (p <0.001). Similar conclusions were evident in a study of patients after AF ablation, where in patients with CHA 2 DS 2 -VASc score of 0 to 1, the presence of CKD was associated with a 14-fold increase in the rate of thromboembolic events. Therefore, all patients with AF with CKD are at increased risk of thromboembolic events.
Obstructive sleep apnea
Obstructive sleep apnea is among the more novel cardiovascular risk factors, associated with a number of cardiovascular diseases, including hypertension, coronary artery disease, myocardial infarction, stroke, or arrhythmias. This translates into elevated risk of stroke seen in, but this seems to be related to several factors, including a prothrombotic state, endothelial dysfunction, intermittent hypoxia, variations in intrathoracic pressure, and recurrent arousals associated with increased heart rate and blood pressure.
The prevalence of obstructive sleep apnea in patients with AF is very high. In the general population, the estimated obstructive sleep apnea prevalence is 2% to 4%, whereas in patients with AF referred for ablation, obstructive sleep apnea was found in nearly 46% of cases. Recently, obstructive sleep apnea and AF, along with erectile dysfunction, have been proposed to be a single clinical entity.
Obstructive sleep apnea (OSA) is directly and independently associated with elevated thromboembolic risk in AF. Our study included 254 patients with AF who underwent polygraphy for a diagnosis of OSA and had their thromboembolic risk assessed using the CHADS 2 and CHA 2 DS 2 -VASc scores. The patients with obstructive sleep apnea had significantly greater CHADS 2 and CHA 2 DS 2 -VASc scores and along with the increasing obstructive sleep apnea severity expressed by greater values of the apnea-hypopnea index, also the mean risk score increased. A study by Yaranov et al found that in patients with CHA 2 DS 2 -VASc score = 0, the presence of obstructive sleep apnea increased the risk of stroke by 62%.
Other clinical risk factors associated with increased thromboembolism
Amyloid is a fibrous protein aggregate composed of various forms of inappropriately folded proteins and polypeptides. It is associated with degenerative diseases of central nervous system and the heart. One of its subforms, serum amyloid protein A, is considered to be an inflammatory cytokine. Elevated serum amyloid protein A levels have been found in patients with AF and are associated with atrial remodeling and inflammatory state and promote vascular thrombosis. This association is confirmed by higher levels of serum amyloid protein A found in patients with venous thromboembolism compared with control group. A different type of the protein, namely β amyloid, is extensively deposited within the walls of small vessels, especially in the elderly. The presence of amyloid angiopathy in the cerebral vessels predisposes not only to greater risk for thromboembolic events but also for intracranial hemorrhage related to anticoagulation.
Also, some myocardial diseases such as obstructive hypertrophic cardiomyopathy (HC) are linked with alterations in coagulation parameters. In patients with obstructive HC, various indicators of the coagulation activation are found. They include elevated levels of plasma fibrinopeptide A and thrombin-antithrombin HI complex. Nearly 15% of patients with HC develop AF, and such patients are at high risk of stroke and thromboembolism. Others have proposed the addition of hyperlipidemia and smoking to CHA 2 DS 2 -VASc, the so-called CHA 2 DS 2 -VASc-HS score, proposed to predict vascular events, not thromboembolism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


