In-stent restenosis (ISR) is a major cause of failure of percutaneous coronary intervention. The efficacy and safety of drug-coated balloon (DCB) in patients with high-risk clinical features are largely unknown. We enrolled 82 consecutive patients at high risk of bleeding with angiographically significant (diameter stenosis ≥50%) ISR of bare metal stent (BMS) or drug-eluting stent (DES), treated with paclitaxel-coated balloon. All patients presented at least one of the following criteria: high bleeding risk, neoplasm, chronic inflammatory disease, and need for noncardiac surgery. Dual antiplatelet therapy was indicated for 4 weeks after the procedure. At angiographic follow-up, overall late lumen loss was 0.24 ± 0.32 mm, with no significant difference between BMS-ISR and DES-ISR (0.25 ± 0.35 vs 0.22 ± 0.30 mm, p = 0.714). The Kaplan-Meier estimate for major adverse clinical events-free survival at 3 years was 81.4% (82.3% in BMS-ISR vs 79.4% in DES-ISR, log-rank p = 0.866). No stent thrombosis has been recorded. In conclusion, the use of paclitaxel-coated balloon seems to be associated with favorable outcomes after percutaneous coronary intervention for BMS-ISR or DES-ISR in patients with high-risk clinical features and could be considered as a reasonable option in the presence of systemic co-morbidities and contraindications to long-term dual antiplatelet therapy.
Drug-coated balloons (DCB) have been proposed as a new treatment for in-stent restenosis (ISR), allowing local delivery of drugs inhibiting neointimal thickening without the need for stent implantation. Currently, the only commercially available DCB are based on paclitaxel, a lipophilic drug targeting smooth muscular cells, which is effectively eluted by DCB with 30- to 60-s inflations. Paclitaxel-coated balloons (PCB) have been tested for the treatment of ISR after BMS implantation, resulting in persistently lower rates of target lesion revascularization (TLR) compared with both uncoated balloons and paclitaxel-eluting stents. Furthermore, PCB have also shown better outcomes compared with uncoated balloons and similar clinical results compared with PES in the treatment of drug-eluting stent (DES) restenosis but inferior angiographic and long-term clinical results compared with everolimus-eluting stents (EES). However, all the studies investigating safety and efficacy of PCB were mainly performed in highly selected populations, with stringent exclusion criteria concerning both clinical and angiographic features. Aim of the present study was to evaluate the efficacy and safety of DCB in a cohort of high-risk patients presenting with ISR of BMS or DES.
Methods
This prospective observational study enrolled consecutive patients at high risk of bleeding who underwent coronary angioplasty for ISR after BMS or DES implantation at Campus Bio-Medico University of Rome, Italy, from January 2011 to February 2014. Eligible patients presented with stable angina, non–ST-segment elevation acute coronary syndrome, or documented silent ischemia, with diameter stenosis ≥50%. Patients were included in the study if they presented at least one of the following features: recent surgery, history of gastrointestinal bleeding, history of hemorrhagic stroke, ischemic stroke in the previous 3 months, need for oral anticoagulation, neoplasm, chronic inflammatory disease, and need for noncardiac surgery. Exclusion criteria were ST-segment elevation myocardial infarction (MI) and hypersensitivity to paclitaxel. The study was approved by the local ethics committee, and written informed consent was obtained from all patients. No external source of funding supported this study.
Cardiac catheterization was performed according to standard practice. Patients received aspirin 250 mg intravenously and clopidogrel 600 mg within 12 hours before the procedure. Glycoprotein IIb/IIIa antagonists were administered at the operator’s discretion. The baseline angiography of the target vessel was performed in at least 2 near-orthogonal views showing the target lesion free of foreshortening and vessel overlap. Three different DCB catheters were used: In.Pact Falcon Paclitaxel-Eluting Balloon (Medtronic Inc., Minneapolis, Minnesota), Pantera Lux Paclitaxel-Coated PTCA Balloon Catheter (Biotronik, Bulach, Switzerland), and Restore Paclitaxel-Eluting Coronary PTCA Catheter (Cardionovum GmbH, Bonn, Germany). The choice of DCB was left at the operator’s discretion in all cases. Balloon catheters were available in length ranging from 10 to 30 mm and diameter ranging from 2.0 to 4.0 mm. In all cases, high-pressure predilation of the target lesion was performed with a nonstudy regular balloon catheter (semi-compliant or noncompliant) to achieve optimal preparation of the lesion before DCB inflation. Maximal balloon-to-artery ratio was 1.26:1 for predilation balloons and 1.19:1 for DCB, respectively. DAPT with aspirin and clopidogrel was indicated for 4 weeks after the procedure.
Angiography was performed before and after all interventions, at 6 months, and at unscheduled occasions using identical projections. Quantitative analysis of the coronary angiographic images was performed in the catheterization laboratory using the CAAS II system (Pie Medical, Maastricht, The Netherlands). Coronary angiograms were analyzed by 2 independent cardiologists (MM and FM). An in-stent analysis (from shoulder to shoulder of the dilated DCB) was performed. Restenosis was defined as recurrent diameter stenosis ≥50% and categorized according to the classification proposed by Mehran et al. Angiographic success was defined as achievement of final residual stenosis <30% by visual estimate and Thrombolysis In Myocardial Infarction (TIMI) flow grade 3.
Clinical follow-up was obtained in all patients through office visits and telephone interviews. Primary end point of the study was late lumen loss (LLL; the difference between the in-stent minimal lumen diameter after the procedure and at 6 months). Secondary end point was the rate of major adverse clinical events (MACE), which included death, MI, and TLR. Cardiac death, TLR, target vessel revascularization, and stent thrombosis were defined according to the Academic Research Consortium recommendations, whereas MI was defined according to the Universal Definition of Myocardial Infarction. TIMI major and minor bleeding was recorded.
Statistical analysis was performed using STATA/IC software, version 12 (STATA Corp., College Station, Texas). Continuous variables are expressed as mean ± SD or median (interquartile range). Categorical variables are reported as frequencies and percentages. Normal distribution was assessed by the Kolmogorov-Smirnov test. Student’s t test or Mann-Whitney test were used to compare continuous variables, as appropriate. Comparisons between categorical variables were evaluated using 2-tailed Fisher’s exact test or Pearson’s chi-square test, as appropriate. Survival was evaluated by Kaplan-Meier method, and comparisons were performed with the log-rank test. A p value <0.05 was considered statistically significant.
Results
We enrolled a total of 82 patients, of which 48 (59%) presented with ISR of a BMS and 34 with ISR of a DES. Baseline clinical and procedural characteristics are listed in Tables 1 and 2 , respectively. High-risk features were active neoplasm in 44 patients, genitourinary bleeding in 6 patients, gastrointestinal bleeding in 4 patients, need for noncardiac surgery in 7 patients, recent surgery in 6 patients, need for oral anticoagulation because of mechanical valve prosthesis in 8 patients, bowel inflammatory disease in 5 patients, and history of hemorrhagic stroke in 2 patients.
| Variable | All patients (n=82) | BMS-ISR (n=48) | DES-ISR (n=34) | p value |
|---|---|---|---|---|
| Mean age (years) | 71±9 | 72±10 | 69±9 | 0.167 |
| Men | 53 (64%) | 32 (67%) | 21 (62%) | 0.647 |
| Diabetes mellitus | 34 (41%) | 20 (42%) | 14 (41%) | 0.965 |
| Hypertension ∗ | 58 (70%) | 37 (77%) | 21 (62%) | 0.133 |
| Dyslipidemia † | 62 (75%) | 38 (79%) | 24 (71%) | 0.373 |
| Cigarette smoke | 39 (47%) | 24 (50%) | 15 (44%) | 0.599 |
| Clinical pattern | 0.671 | |||
| Stable angina pectoris | 32 (39%) | 18 (37%) | 14 (41%) | |
| NSTE-ACS | 19 (23%) | 10 (21%) | 9 (26%) | |
| Silent myocardial ischemia | 31 (38%) | 20 (42%) | 11 (32%) | |
| Prior myocardial infarction | 38 (46%) | 25 (52%) | 13 (38%) | 0.215 |
| Prior coronary artery by-pass graft | 3 (4%) | 1 (2%) | 2 (6%) | 0.567 |
∗ Systolic blood pressure: >140 mm Hg and/or diastolic blood pressure >90 mm Hg or current antihypertensive treatment.
| All patients (n=82) | BMS-ISR (n=48) | DES-ISR (n=34) | p value | |
|---|---|---|---|---|
| Multivessel coronary disease | 18 (22%) | 11 (23%) | 7 (21%) | 0.802 |
| Treated coronary vessel | 0.279 | |||
| Left main | 1 (1%) | – | 1 (1%) | |
| Left anterior descending | 47 (57%) | 25 (52%) | 22 (65%) | |
| Left circumflex | 17 (21%) | 12 (25%) | 5 (15%) | |
| Right | 16 (19%) | 11 (23%) | 5 (15%) | |
| Saphenous vein graft | 1 (1%) | 0 (0%) | 1 (1%) | |
| Time from stent implantation (months) | 7.6±5.3 | 6.7±2.2 | 9.1±6.1 | 0.014 |
| Restenosis | 0.842 | |||
| Type 1 | 58 (71%) | 34 (71%) | 24 (71%) | |
| Type 2 | 19 (23%) | 11 (23%) | 8 (24%) | |
| Type 3 | 4 (5%) | 2 (4%) | 2 (5%) | |
| Type 4 | 1 (1%) | 1 (1%) | – | |
| Recurrent restenosis | 8 (10%) | 3 (6%) | 5 (15%) | 0.266 |
| N° of DBC used per patient | 1.04±0.19 | 1.02±0.14 | 1.06±0.24 | 0.373 |
| Drug-coated balloon | 0.981 | |||
| In.Pact Falcon | 22 (27%) | 13 (27%) | 9 (26%) | |
| Pantera Lux | 20 (24%) | 12 (25%) | 8 (24%) | |
| Restore | 40 (49%) | 23 (48%) | 17 (50%) | |
| DCB diameter (mm) | 3.21±0.47 | 3.24±0.39 | 3.11±0.51 | 0.195 |
| DCB length (mm) | 16.1±4.2 | 15.8±4.3 | 16.5±4.7 | 0.487 |
| Maximal inflation pressure (mmHg) | 13.1±2.3 | 13.2±2.4 | 13.0±2.2 | 0.702 |
| Inflation time, sec | 53±9 | 52±8 | 54±10 | 0.318 |
| Procedural success | 82 (100%) | 48 (100%) | 34 (100%) | 1.000 |
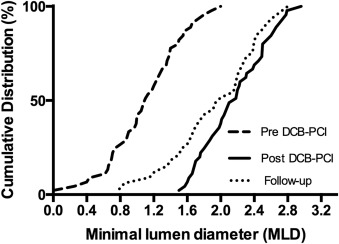
A total of 68 patients (82.9%) underwent follow-up angiography. Of these, 40 were in the BMS-ISR group and 28 in the DES-ISR group (p = 0.907). Follow-up angiography was unplanned in 12 patients (17.6%), driven by return of anginal symptoms. Interobserver variability was 0.02 ± 0.18 mm for RVD, 0.03 ± 0.15 mm for minimal lumen diameter, and 0.38 ± 4.1% for diameter stenosis. Overall, LLL was 0.24 ± 0.32 mm, with no significant difference between the BMS-ISR and the DES-ISR groups (0.25 ± 0.35 vs 0.22 ± 0.30 mm, p = 0.714; Table 3 ). Cumulative frequency distribution of minimal lumen diameter before percutaneous coronary intervention, after the procedure and at follow-up angiography, is shown in Figure 1 .
| Variable | All patients (n=82) | BMS-ISR (n=48) | DES-ISR (n=34) | p value |
|---|---|---|---|---|
| Pre-procedure | ||||
| RVD (mm) | 2.48±0.46 | 2.50±0.44 | 2.45±0.50 | 0.633 |
| MLD (mm) | 1.11±0.44 | 1.18±0.48 | 1.00±0.36 | 0.068 |
| Diameter stenosis (%) | 72.8±13.5 | 72.2±11.2 | 73.9±16.9 | 0.585 |
| Post-procedure | ||||
| RVD (mm) | 2.50±0.46 | 2.52±0.44 | 2.47±0.50 | 0.633 |
| MLD (mm) | 2.17±0.39 | 2.22±0.39 | 2.14±0.39 | 0.363 |
| Diameter stenosis (%) | 18.3±8.1 | 17.2±8.4 | 20.2±7.4 | 0.098 |
| Follow-up | ||||
| Available data | 68 (83) | 40 (83) | 28 (82) | 0.907 |
| RVD (mm) | 2.50±0.45 | 2.51±0.45 | 2.48±0.44 | 0.786 |
| MLD (mm) | 1.95±0.54 | 1.97±0.43 | 1.93±0.62 | 0.754 |
| Diameter stenosis (%) | 34.9±22.3 | 32.2±18.4 | 36.8±25.0 | 0.385 |
| Late lumen loss (mm) | 0.24±0.32 | 0.25±0.35 | 0.22±0.30 | 0.714 |
No inhospital MACE was recorded. DAPT was withdrawn 4 weeks after the procedure in 78 patients (95.1%), and thereafter, only 1 antiplatelet drug (aspirin in 67 patients and clopidogrel in 11 patients) was continued indefinitely. The duration of DAPT in the remaining 4 patients ranged from 6 to 13 months. All 8 patients on oral anticoagulation because of mechanical valve prosthesis were kept on DAPT for 4 weeks, and thereafter, aspirin was continued indefinitely. Clinical follow-up was obtained in all patients ( Table 4 ). Median duration of follow-up was 18 months, ranging from 3 to 45 months. Three patients (3.7%) died from noncardiac death: 2 patients died from neoplasm complications and 1 died during nonvascular abdominal surgery. No cardiac death, MI, or stent thrombosis was recorded. TLR was performed in 8 patients (9.8%), whereas in total 10 patients (12.1%) underwent target vessel revascularization. The number of patients presenting TLR according to the type of DCB used is reported in Figure 2 . The Kaplan-Meier estimate for MACE-free survival at 3 years was 81.4%, with no significant differences between BMS-ISR and DES-ISR (82.3% vs 79.4%, log-rank p = 0.866; Figure 3 ). During follow-up, TIMI major bleeding occurred in 3 patients (3.7%) and TIMI minor bleeding occurred in 5 patients (6.1%). TIMI major bleeding occurred in all cases after DAPT withdrawal, whereas TIMI minor bleeding occurred in 2 cases during DAPT; however, antiplatelet treatment was not interrupted in any case.



