Management of acute ischemic stroke
Introduction
Epidemiology
Worldwide, stroke is the second most common cause of death after coronary heart disease (CHD)1 and accounts for 4.38 million deaths annually, with almost three million of those in developing countries. Most strokes are not fatal2 and consequently mortality data underestimate the true global burden of stroke which is chronic disability.3 Stroke is the sixth most common cause of disability-adjusted life years (DALYs).4 More than 80% of strokes occur in the 14% of the population aged 65 and older5 and 20–25% of individuals will have a stroke if they live until 85 years.6 Consequently the incidence of stroke and socioeconomic burden is expected to increase globally and particularly in Western countries, where there is expected to be a shift in the age distribution of the population towards older ages. By 2030 stroke is projected to be the fourth most common cause of reduced DALYs.7
Estimates of the incidence of stroke come from a number of population-based epidemiologic studies. One of the largest studies to examine the incidence of vascular disease including stroke is the Oxford Vascular Study (OXVASC) in which > 91 000 individuals were followed over a three-year period in Oxfordshire, United Kingdom (UK). The rate per 1000 population per year of first-ever incident cerebrovascular events was 2.27 (95% confidence interval (CI) 2.09–2.45) which was higher than event rates in the coronary and peripheral vascular beds5 (Table 61.1). Event rates rose steeply with age with 80% of strokes occurring in the 14% of the study population aged 65 and older. Long-term trends in stroke incidence have been reported over a 20-year period in Oxfordshire. The age-specific incidence of stroke has fallen by 40% largely due to increased use of preventive medications and risk factor modifications.8
Table 61.1 Incidence of first and recurrent stroke compared to ischemic heart disease and peripheral vascular disease in O xford V ascular S tudy population5
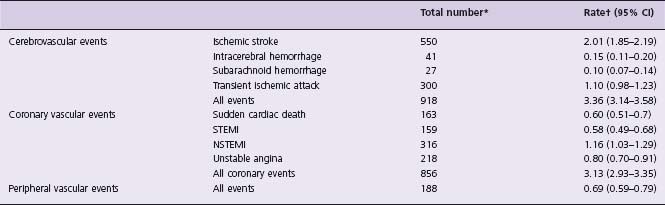
STEMI, ST segment elevation acute myocardial infarction; NSTEMI, non-ST segment elevation acute myocardial infarction.
* Number of events during 3 years.
† Number of events per 1000 population per year.
Reproduced from Donnan and colleagues95 with permission.
Nomenclature, definitions
Stroke is divided broadly into either ischemic or hemorrhagic types with ischemic strokes accounting for the majority of stroke (>80%).9 The treatment of these stroke subtypes is dramatically different and as a result, one of the first steps in managing stroke patients is to evaluate them for the presence of hemorrhage. Clinical features of hemorrhagic and ischemic stroke overlap and as a result, the distinction is often made with hyperacute computed tomography (CT) imaging. Specialized magnetic resonance imaging (MRI) sequences are more sensitive than CT to acute and chronic hemorrhage and better at detecting early ischemia and are becoming more commonly used for the early diagnosis of stroke.10
Ischemic strokes can be further classified based on clinical findings and the results of investigations into subtypes. The value of identifying stroke subtype is that identifying the most likely mechanism of vessel occlusion helps guide early treatment and subsequent investigations. The Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria is one of the most widely employed classification systems for ischemic stroke.11 Ischemic stroke, based on the presumed pathophysiologic mechanism of occlusion, is subdivided into small vessel occlusion (“lacunes”), large artery atherosclerosis (embolus or thrombosis) and cardioembolic. The TOAST criteria also recognize that a proportion of ischemic stroke will remain cryptogenic in etiology or will result from some combination of mechanisms or other causes such as dissection or a vasculitis.
There are two subtypes of hemorrhagic stroke: intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH). Primary ICH is more common (about 8% of total strokes) and is most often caused by hypertensive small vessel disease which causes the rupture of small lipohyalinotic aneurysms.12 SAH accounts for about 5% of strokes and is usually caused by the rupture of saccular aneurysms within the subarachnoid space. The acute management of ICH frequently involves admission to an intensive care unit and management of increased intracranial pressure, a discussion of which is beyond the scope of the current chapter (see American Heart Association guidelines for detailed review13). With the exception of a recently reported negative Phase III trial of hemostasis with recombinant activated factor VII,14 treatments for ICH have not been subjected to large randomized trials. There are no therapies for ICH with Level A evidence and in general, the evidence guiding therapy for ICH is less established. Acute ischemic stroke treatments with Level A evidence are summarized in Table 61.2.
Table 61.2 Acute stroke management strategies of proven benefit (Level A)

RRR, relative risk reduction; ARR, absolute risk reduction; NNT, numbers needed to treated; IV tPA, intravenous tissue plasminogen activator; ASA, acetylsalicylic acid; MCA, middle cerebral artery.
Acute stroke management
Prehospital management
The treatment of acute ischemic stroke (AIS) is exquisitely time sensitive and the speed and accuracy of evaluating and managing patients are important in optimizing patient outcomes. Based on data extrapolated from experimental studies of ischemic stroke, with each minute that passes there is a loss of 1.9 million neurons.15 Accordingly, timely reperfusion of ischemic brain is the primary goal of most acute stroke therapies.
The narrow time window for the delivery of reperfusion therapies such as intravenous (IV) recombinant tissue plasminogen activator (tPA) necessitates the development of stroke-specific prehospital algorithms to ensure the timely transport of patients to hospitals capable of providing acute stroke care. The most time-efficient means for stroke patients to seek medical care is by activating Emergency Medical Services (EMS) by calling 911 or the equivalent “ immediate ” EMS response phone service. Compared to patients who contact their primary physician or hospital directly, stroke patients who call EMS are more likely to arrive within the three-hour time window.16 Currently only about half of patients with signs and symptoms of acute stroke will use EMS to first access medical care.17 The decision to call 911 either by patients or other bystanders is influenced by the knowledge that stroke is a serious and treatable disease.18 There is evidence that patients transported to hospitals through the activation of emergency stroke pathways receive neurologic attention sooner, more frequently are treated with tPA, and have better outcomes19 (Level C1).
Early recognition of stroke by EMS paramedics helps ensure that patients are provided with appropriate initial stabilization and therapy, and advance notice is provided to the receiving emergency department. Diagnosis of stroke in the prehospital setting can be facilitated by the use of validated prehospital screening tools (Class I, Level B) such as the Los Angeles Prehospital Stroke Screen20 and the Cincinnati Prehospital Stroke Scale.21 Once a diagnosis of stroke is made, patients with acute symptoms that may eligible for fibrinolytic therapy can then be transported to the closest facility that provides emergency stroke care. In rural areas where long distances prevent timely transport of patients to specialized stroke centers, telestroke systems can facilitate the safe delivery of intravenous tPA administration22–24 (Class IIa, Level B).
Emergency management
The initial management of acute stroke patients most often occurs in the emergency department (ED) of hospitals. A number of conditions can mimic stroke including seizures, tumors, infection, hypoglycemia and other metabolic abnormalities. Stroke mimics are common and 13–31% of patients initially diagnosed with acute stroke in the emergency room have other conditions.25,26 Early brain imaging of stroke patients is required to help make decisions about the emergency management. A non-contrast enhanced CT (NCCT) scan is recommended to exclude non-vascular causes of neurologic symptoms and identify brain hemorrhage (Class I, Level A). NCCT will often show early signs of brain ischemia and identify arterial occlusion; however, the interobserver agreement for these early infarction signs on NCCT is general poor (κ statistic range 0.14–0.78).27 NCCT (performed within 14 hours of symptom onset) will reveal brain parenchymal abnormalities in up to 94% of ischemic stroke patients28 ; however, the sensitivity decreases at earlier time points. In the first six hours the sensitivity of NCCT is 66% (range, 20–87%) with a specificity of 87% (range, 56–100%).27 At under three hours, which is the time window for treatment with intravenous fibrinolytics, the diagnostic sensitivity of NCCT has been reported as low as 7% (range 3–14%).10
The sensitivity of MRI with diffusion-weighted susceptibility images (DWI) is greater than NCCT for the detection of acute stroke (83% vs 26%).10 DWI MRI sequences measure the degree of free diffusion of water molecules. With cerebral ischemia and subsequent cytotoxic edema, water diffusion becomes restricted and will result in abnormal DWI signal29 that can be seen within minutes of stroke onset.30 Case–control studies have suggested that the use of MRI with DWI may improve the safety and efficacy outcomes of fibrinolytic therapy31 ; however, at present there is insufficient evidence to recommend the routine use of MRI for the initial management of acute stroke (Class II, Level C).
Early infarction signs such as extent of brain parenchymal hypoattenuation, hyperattenuation within the intracranial artery, and swelling in the affected hemisphere increase the risk of poor functional outcome (overall odds ratio (OR) 3.11; 95% CI 2.77–3.49) but do predict response to fibrinolytic therapy.27 Other than the identification of intracerebral hemorrhage, no finding on NCCT should preclude treatment of AIS patients with tPA within three hours of symptom onset (Class IIb, Level A).
General supportive care
There are few data from clinical trials to guide initial medical care of acute stroke patients. It is generally agreed that ventilatory assistance should be provided to patients with decreased level of consciousness or bulbar dysfunction compromising the airway. Supplemental oxygen should be provided to prevent hypoxia but in general most stroke patients do not need or benefit from routine oxygen supplementation.32 Furthermore, increasing brain tissue oxygenation with hyperbaric oxygen therapy has been the focus of a Cochrane review and the authors concluded that there was no evidence of clinical benefit.33
Fever in the setting of acute stroke has been consistently associated with stroke severity, infarct size and poor neurologic outcomes34,35 and should be treated with antipyretics such as acetaminophen (Class I, Level C). In experimental studies, small elevations in ischemic brain temperature have a large impact on the extent of ischemic neuronal injury.36 A few small trials have examined whether prophylactic antipyretic use improves clinical outcomes. These studies have shown a small mean decrease in body temperature in treated patients but without any clear benefit on clinical outcomes. There is both experimental and clinical evidence to suggest that more aggressive lowering of body temperature (induced hypothermia) can protect the brain after cardiac arrest.37 The value of induced hypothermia in stroke is not yet established but is the focus of ongoing investigations.
Hyperglycemia is commonly detected in acute stroke patients38 and has been associated with poor outcomes and increased odds of symptomatic intracerebral hemorrhage (OR 1.75 per 100 mg/dL increase in admission glucose, 95% CI 0.61–0.95), independent of fibrinolytic treatment.39 Hyperglycemia is thought to increase hemorrhage by provoking anaerobic metabolism, lactic acidosis, and free radical production, resulting in membrane lipid peroxidation and cell lysis in ischemic brain tissue.40 A randomized clinical trial (GIST-UK) of aggressive treatment of hyper-glycemia in acute stroke with glucose-potassium-insulin infusions found lower blood pressures and blood glucose in treated patients but no differences in stroke outcomes41 (Level A). In all stroke patients, monitoring of blood glucose is recommended and persistent extreme hyperglycemia should be treated with insulin (Class II, Level C). Hypoglycemia can result in brain injury and mimic stroke symptoms and should be corrected as part of the emergency management of stroke patients (Class I, Level C).
Acute management of blood pressure
The goal of blood pressure (BP) management in acute stroke is to maximize perfusion to the ischemic penumbra while minimizing the risk of hemorrhagic transformation. Both high and low blood pressures are associated with poor outcomes.42 Data from the IST trial showed that early death increased by 17.9% for every 10 mm Hg of systolic blood pressure (SBP) below 150 mm Hg and by 3.8% for every 10mmHg SBP above 150mmHg, suggesting a “U shaped ” curve.43 The optimal blood pressure in the setting of acute stroke remains poorly defined with little clinical trial evidence to guide decisions about blood pressure thresholds. Current guidelines for blood pressure management are largely empirically derived.44
BP elevation is often seen in the first minutes to hours after the onset of focal cerebral ischemia45 and represents a compensatory reaction to vascular occlusion. Targets for the treatment of hypertension depend on whether the patient is a potential candidate for fibrinolytic therapy. A systolic blood pressure >185mmHg or a diastolic blood pressure >110mmHg is a contraindication to intravenous administration of tPA, which is based on the criteria for the NINDS trial46 rather than experimental data. Patients with blood pressures above this level should be treated prior to starting lytic therapy (Class I, Level B).
There is a theoretic basis for not being overly aggressive with BP lowering in the hyperacute phase of ischemic stroke. Cerebral autoregulation is disrupted by acute ischemia and subsequent tissue acidosis and in turn leads to maximal vasodilation.47 As a result, with vascular occlusion, overly aggressive treatment of blood pressure could reduce cerebral blood flow (CBF) through stenotic and collateral vessels48 and cause tissue in the ischemic penumbra to progress to infarction. As a result, AIS patients not receiving tPA therapy should be treated with antihypertensive medications only when blood pressure exceeds 220/120mmHg.44 This blood pressure upper limit corresponds to a mean arterial pressure of around 150 mm Hg, which is the normal upper limit of cerebral autoregulation (Class II, Level C).
In acute stroke, tolerating elevated blood pressures in order to maintain CBF (“ permissive hypertension ”) needs to be balanced with the potentially harmful effects of severe hypertension. Acute blood pressure elevation in stroke may increase the risk of ICH and worsen cerebral edema. However, the benefits of early BP reduction have yet to be confirmed in large clinical trials. Only five trials and a total of 218 patients were identified in a recent systematic review of blood pressure alterations in acute stroke and the conclusion was that data were too limited to assess the effect of BP modulation on clinical outcomes.49
Intravenous thrombolysis
Within the first hours after the onset of stroke symptoms, most patients with persistent arterial occlusion will have brain tissue that is hypoperfused and functionally inactive.50 Hypoperfused brain tissue that has not been irreversibly damaged is termed ischemic penumbra. Penumbral tissue is present in almost all stroke patients at under three hours but after three hours the number of patients with salvable penumbra rapidly diminishes.51
Early vessel recanalization and cerebral reperfusion is goal of most acute stroke therapies and it is the most effective means to salvage penumbral tissue and improve clinical outcomes. A meta-analysis of pooled data from 53 studies of acute stroke therapies that reported recanalization rates found that good functional outcomes at three months were more frequent in recanalized than in non-recanalized patients (OR 0.24, 95% CI 0.16–0.35) with no difference in symptomatic hemorrhage52 (Level B).
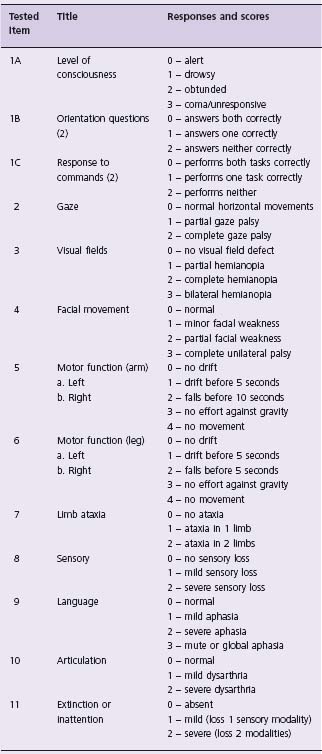
Currently the only approved and recommended reperfusion therapy for AIS is thrombolysis with intravenous tPA administered within three hours of symptom onset (Class I, Level A). Intravenous tPA was approved for use in AIS by the US Food and Drug Administration in 1996 based partly on the National Institute of Neurologic Disease and Stroke (NINDS) tPA Stroke Study.46 In the study, 624 patients with ischemic stroke were treated with placebo or tPA (0.9 mg/kg IV, maximum 90 mg; 10% given as a bolus with the remainder given over one hour) within three hours of symptom onset and almost half of patients were treated within 90 minutes. The NINDS study was conducted in two parts. The primary endpoint in part I was neurologic improvement in 24 hours as indexed by an improvement of ≥ 4 points on the National Institutes of Health Stroke Scale (NIHSS) (Table 61.3) or complete neurologic recovery. Part II of the study used a global test statistic to assess clinical outcome at three months, with favorable outcome defined as a complete or nearly complete neurologic recovery. The trial showed no group difference in percentages of patients with neurologic improvement at 24 hours. At three months, however, tPA treatment resulted in a 32% relative (12% absolute) increase in the proportion of patients with minimal or no disability.46 The major risk of treatment with tPA is symptomatic hemorrhage, which in the NINDS trial occurred in 6.4% of treated patients compared to 0.6% in the placebo group. Despite the increased hemorrhage risk, there was no difference in the three-month mortality between the tPA-treated and placebo groups (17% vs 20%, respectively). Recently a large prospective, open-label, observational study of 6438 patients from 14 countries confirmed that tPA is safe, with hemorrhage rates comparable to clinical trials, when used within three hours of stroke onset following protocols, even in centers with little prior experience in treating acute stroke patients.53
Table 61.3 National Institutes of Health Stroke Scale (NIHSS) used to measure the severity of stroke deficit. Total NIHSS score is calculated by adding scores on the 11 items
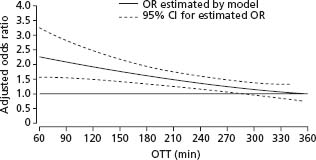
In addition to the NINDS trial, there have been a number of other trials of intravenous fibrinolytic agents in stroke. These trials all had important differences from the NINDS trial, including the use of higher doses of tPA,54 the randomization of patients beyond three hours,54–57 and treatment with other fibrinolytics such as streptokinase along with antithrombotic drugs.58–60 Streptokinase trials were halted prematurely due to high hemorrhage rates and its use is not recommended (Class III, Level A). Intention to treat analysis of data pooled from randomized trials of tPA in stroke patients treated up to six hours (NINDS, ECASS-I, ECASS-II and ATLANTIS) has highlighted the important relationship between time to initiate treatment and clinical outcomes.54–57 Combined data from 2775 stroke patients treated in more than 300 hospitals in 18 countries was used to analyze the relationship between tPA benefit and time to treatment.61 This study showed that the odds ratio for favorable outcome at three months and adjusted for baseline clinical features decreases as time to treatment increases (Fig. 61.1). The odds of a favorable outcome was greatest in the 0–90 minute interval (1.2, 95% CI 1.8–4.5). The beneficial effect of tPA extended beyond three hours to 270 minutes at which point the lower confidence interval for the odds ratio crosses unity (see Fig. 61.1).
Figure 61.1 Model estimating odds ratio for favorable outcome at 3 months in rt-PA treated patients compared with controls by OTT. Adjusted for age, baseline glucose concentration, baseline NIHSS measurement, baseline diastolic blood pressure, previous hypertension, and interaction between age and baseline NIHSS measurement. (From Hack et al.61 with permission).
The recently completed ECASS-3 trial provides new data on the tPA treatment in an extended time window.61a The trial randomized ischemic stroke patients (n= 821) between 3 and 4.5 hours after symptom onset to treatment with IV tPA or standard medical care. The tPA dosing regimen and enrollment criteria were similar to the current guidelines for treating stroke patients within 3 hours. More patients treated with tPA had good outcomes (no or minimal disability) than with placebo (52.4% vs 45.2%; odds ratio, 1.34; 95% CI, 1.02 to 1.76; P= 0.04) although the degree of benefit was less than that of patient enrolled from 0 to 3 hours in the NINDS study. The tPA treated group had higher rates of symptomatic intracranial hemorrhage (2.4% vs 0.2%) but this did not result in any increase in mortality. ECASS-3 provides further evidence that although the benefits of tPA are attenuated at later time points, IV tPA therapy can be administered safely from 3 to 4.5 hours in select patients and improves clinical outcomes (Class I, Level B).61b
Recent studies have examined whether specialized stroke MRI sequences that include DWI and images of brain perfusion can identify ischemic stroke patients with penumbral tissue who may benefit from perfusion with IV tPA beyond three hours.62,63 These studies show that patients with large regions of hypoperfusion and relatively small areas of tissue infarction (i.e. large amounts of penumbral tissue) may safely be given tPA beyond three hours. The subset of patients with large amounts of penumbral tissue and early reperfusion shows the greatest improvement in clinical outcomes.63 The value of MRI selection of stroke patients for IV tPA therapy beyond three hours has yet to be replicated in larger Phase III trials.
Other reperfusion therapies that are currently being evaluated in clinical trials include alternative fibrinolytic agents such as reteplase, desmoteplase, and tenecteplase, and augmenting IV tPA with transcranial Doppler.64 At present, there are insufficient data to support the use of any of these reperfusion approaches (Class III, Level C).
Intra-arterial thrombolysis
Endovascular methods of achieving vessel recanalization in AIS include mechanical and pharmacologic approaches.65 Intra-arterial (IA) delivery of fibrinolytic agents offers the potential advantage of more rapid removal of thrombus compared to intravenous approaches as agents are infused at high concentration in close proximity to sites of occlusion with a microcatheter system. IA therapies, at least in theory, may expand the time window for reperfusion therapy by reducing or eliminating systemic exposure to fibrinolytic agents.
The only randomized trial to assess the efficacy and safety of IA fibrinolytic therapy was the Prolyse in Acute Cerebral Thromboembolism Trial (PROACT-II).66 In PRO-ACT-II, 180 patients with acute stroke of less than six hours and angiographically confirmed occlusion of the middle cerebral artery (MCA) were randomized to receive IA recombinant prourokinase (r-proUK) plus heparin or heparin alone. The primary outcome was the proportion of patients with mild or no neurologic disability at 90 days. Based on an intention to treat analysis, 40% of r-proUK treated patients had good outcomes, which was significantly better than the 25% of control patients. Rates of partial or full recanalization were also markedly higher in the r-proUK group than controls (66% versus 18%). Mortality rates were around 25% for both groups. Symptomatic hemorrhage rates were, however, higher in the r-proUK patients compared to controls (10% vs 2% respectively).
The only other randomized data of IA fibrinolytics come from the recently published MELT trial.67 In this study, conducted in Japan prior to the availability of IV tPA, ischemic stroke patients presenting within six hours were randomized to IA UK or placebo. The trial was halted prematurely largely over ethical concerns about the randomization once IV tPA was approved for use in Japan. The incomplete MELT dataset, however, when pooled with other trials of r-proUK, further supports a benefit of IA fibrinolytics in AIS.68
In addition to pharmacologic approaches to recanalization, there have been a number of endovascular approaches to treat intracranial or extracranial occlusions that do not involve the delivery of fibrinolytics (see Molina and Saver65 for review). At present, the only devices approved by the FDA for the revascularization of patients with AIS are the Merci Retriever device (Concentric Medical) and the Penumbra System (Penumbra Inc.) aspiration catheter. While these devices can achieve high rates of vessel recanalization when used in AIS,69,70 they lack any evidence of improved clinical outcomes from randomized controlled trials.
The treatment of acute stroke with endovascular therapies suffers a number of important limitations that prevent their widespread adoption. Endovascular procedures require substantially more infrastructure support, there are insufficient numbers of neurointerventionalists, treatment is limited to more proximal vessel occlusions, and time to initiate therapy is longer than IV fibrinolysis. Current guidelines recognize the value of IA fibrinolysis as a treatment option for patients with major anterior circulation stroke within six hours of symptom onset and up to 24 hours for vertebrobasilar occlusion (Class I, Level B). IA therapies should not take precedence over the administration of IV tPA to eligible patients.
Anticoagulants
There is no evidence that urgent anticoagulation with either unfractionated or low molecular weight heparin (LMWH) reduces the rate of recurrent stroke, improves neurologic outcomes or can be used either along with or in place of IV tPA (Class III, Level A). The largest study of anticoagulation in AIS was the International Stroke Trial (IST).71 This was a randomized, open-label trial, which compared the safety and efficacy of acetylsalicylic acid (ASA) and subcutaneous heparin (5000 or 12 500 IU) in 19 435 patients. Neither heparin regimen offered any clinical advantage over ASA. The higher dose of heparin was associated with an excess of morbidity and mortality. Post hoc analysis of the IST data examined stroke patients with atrial fibrillation and presumed cardiac embolism. Even in this subgroup of patients, the absolute risk of recurrent stroke in the first 14 days was relatively low (3.9%) and there was no net benefit to use of heparin.72 Other trials have similarly found no benefit of emergency anticoagula-tion for AIS behond prevention of deep vein thrombosis and pulmonary embolism.73
Antiplatelet agents
ASA has been evaluated for the treatment of AIS in two large randomized controlled trials (CAST and IST) and one smaller trial (MAST).58,71,74 In the CAST trial (n= 21 106), patients with acute ischemic stroke were randomized within 48 hours to ASA or placebo. There was a small but significant reduction in mortality amongst ASA-allocated patients (relative risk reduction (RRR) 14%, absolute risk reduction (ARR) 0.6%) and patients treated with ASA had significantly fewer recurrent strokes than patients in the placebo arm (1.6% vs 2.1%, respectively). ASA was associated with slightly more (0.2%) recurrent hemorrhagic strokes but this did not affect overall mortality, as ASA patients were less likely to be dead or dependent at discharge (11.4 fewer per 1000).
Meta-analysis of nine trials (n= 41399) confirmed that acute ASA treatment was associated with a significant decrease in death or dependency at the end of follow-up (OR 0.94; 95% CI 0.91–0.98) and increased odds of making a complete recovery from the stroke (OR 1.06; 95% CI 1.01–1.11).75 For every 1000 ischemic stroke patients treated with ASA within 48 hours, there were 13 more patients alive and independent at follow-up and 10 additional patients made a complete recovery. In addition, per 1000 ASA treated patients there was reduction of seven recurrent ischemic strokes and one pulmonary embolus but at a cost of two excess symptomatic intracranial hemorrhages. Based on these data, ASA is recommended for all ischemic stroke patients within 48 hours of stroke onset provided there are no strong contraindications, and hemorrhagic stroke has been excluded (Class I, Level A). In patients treated with fibrinolytic therapy, ASA should not be given in the first 24 hours.
Neuroprotection
The development of neuroprotective therapies that attenuate the cascade of pathophysiologic events that occur with ischemia in acute stroke has been disappointing. A large number of neuroprotective agents have been developed based on animal models of focal ischemia but all have failed in translation to clinical practice.76 Most recently, the results of a large trial using NXY-059 was reported. The initial NXY-059 trial suggested the drug was safe and effective in improving stroke outcomes77 but a subsequent Phase III trial found no evidence of efficacy.78 The failure to develop neuroprotective agents using current paradigms has led researchers to explore different strategies such as prehospital delivery of neuroprotective drugs.79 Currently, no neuroprotective therapy is recommended for treating stroke (Class III, Level A).
Preventing early complications
Stroke care units
The intervention that has the greatest potential to improve outcomes of stroke patients is the establishment of specialized stroke care units (SCU). A recent Cochrane analysis pooled data from 31 trials and a total of 6936 patients examining the benefits of routine management of stroke patients in SCUs.80 Stroke patients who received organized care in a SCU had significantly lower odds of being dead or dependent at follow-up (OR 0.82; 95% CI 0.73–0.92; P= 0.001) than patients receiving care on general medical wards. Subgroup analyses showed that stroke patients benefit regardless of sex, age or stroke severity. Based on data extrapolated from a community-based epidemiologic study, it is estimated that the establishment of SCUs has the potential to prevent death and disability for 50 patients per 1000 strokes compared to six per 1000 for tPA treatment and four per 1000 with ASA therapy.81
The precise components of an SCU that account for the benefit in functional outcomes are not well delineated. It is established that SCUs that occupy a separate physical space are associated with better outcomes than care provided by mobile stroke teams in general wards.82 Likely interventions that are important for improved outcomes include early mobilization, better blood pressure control, and prevention of complications such as deep venous thrombosis through closer adherence to current evidencebased guidelines. All stroke patients should be admitted to SCUs (Class I, Level A). Stroke units ideally should be geographically distinct, with well-defined protocols that address common problems such as early mobilization, screening for dysphagia, treatment of infections with antibiotics, and subcutaneous administration of anticoagulants for deep vein thrombosis (DVT) prophylaxis.
Venous thromboembolism
Venous thromboembolism is an important cause of morbidity in stroke patients. The risk of venous thromboembolism in patients with AIS approaches that of surgical patients and without thromboprophylaxis, about 20% of patients will develop pulmonary embolus (PE) and 1–2% of stroke patients will have a fatal PE. Unfractionated heparin is associated with an 81% reduction in deep vein thrombosis and 58% reduction in PE with a small increased risk of hemorrhagic transformation of the infarct.83 The recently completed PREVAIL trial showed that thromboprophylaxis with LMWH may further reduce the risk of venous thromboembolism by 43% compared to unfraction-ated heparin; however, most (95%) of the DVT detected in the trial were asymptomatic.84 Current guidelines recommend thromboprophylaxis for all ischemic stroke patients with anticoagulants (Class I, Level A) although there is still controversy about whether LMWH or unfractionated heparin is superior.
Acute neurologic complications
Many patients with ischemic stroke will worsen during the first 24–48 hours after stroke onset. The main neurologic causes of early worsening are the development of intracerebral hemorrhage and space-occupying cerebral edema and, less commonly, seizures. The risk of hemorrhagic transformation of ischemic stroke is increased with fibrinolytic drugs, anticoagulation or antiplatelet agents. Hemorrhage after ischemic stroke can be petechial, which is often asymptomatic, or result in hematoma formation that increases intracranial pressure and results in neurologic decline. There are currently no proven therapies for the treatment of hemorrhagic transformation. Seizures occur in about 3% of stroke patients within the first 24 hours.85 Prophylactic anticonvulsant therapy is not recommended (Class III, Level C).
About 10% of patients with supratentorial infarction will develop lifethreatening, space-occupying brain edema (“ malignant infarction ”) usually on the second to fifth day after stroke.86 The prognosis of patients with malignant MCA territory infarctions is poor, with mortality rates of around 80%87 and no effective medical therapy. There is evidence from three European randomized trials (DECIMAL, DESTINY and HAMLET) that suggests that decompressive surgery improves functional outcomes in patients with malignant MCA infarction88–90 (Level A). Pooled data from these three trials (n= 93) showed that patients in the decompressive surgery group (treated within 48 hours, mean age 45) were more likely than controls to survive their stroke, free of severe disability (75% vs 24%; pooled ARR 51%; 95% CI 34–69).91 Surgery in these trials was performed within 48 hours of stroke onset and the NNT= 4 for survival with a moderate deficit or better. These results highlight that decompressive hemicraniec-tomy increases survival at a cost of more survivors with at least moderate deficits. The timing of surgery and the clinical characteristics of patients ’ most likely to benefit are still not well established. Decompressive hemicraniectomy is usually reserved for younger patients and after discussion about the potential outcomes, including survival with moderate to severe disability (Class II, Level B).
The only other clear indication for surgery in acute stroke is for space-occupying cerebellar infarction (Class I, Level B). In about 11–25% of cerebellar stroke the edema becomes space occupying within the posterior fossa, and brainstem compression and acute obstructive hydrocephalus result.92 The indications for surgical intervention in cerebellar strokes are a decreased level of consciousness and/or clinical signs of brainstem compression. The management involves the insertion of an external ventricular drain (EVD) and/or suboccipital decompressive craniectomy; however, the optimal procedure is controversial.93 While the efficacy is not supported by clinical trials, case series have established decompressive surgical evacuation of space-occupying cerebellar infarction as a potentially life-saving procedure.94
Conclusion
Over the past decade, there have been significant advances in our knowledge of the epidemiology, pathophysiology, and treatment of acute stroke. Management of acute stroke has emerged from a long period of therapeutic nihilism, largely the result of the recognition that, with emergent evaluation and treatment, ischemic brain can be salvaged and patient outcomes improved.
Perhaps the most significant advance in acute stroke management is the development of specialized stroke units. From a population health perspective, stroke care units have the potential to be widely implemented and benefits can extend to almost all stroke patients. Stroke units provide an opportunity to implement evidencebased treatment pathways focused on salvaging penumbral tissue, preventing post-stroke complications, initiating secondary prevention regimens, and promoting recovery through early rehabilitation. Compared to stroke units, no other acute stroke treatment strategy has the potential to have such a large impact on saving lives and reducing long-term dependency.
Almost all patients with AIS can benefit from the early administration of ASA. ASA is the only medical therapy proven to improve stroke outcomes, prevent recurrent stroke, and reduce stroke mortality. In comparison, the narrow time window for administration of IV tPA limits the potential benefit to only a small percentage of stroke patients. IV tPA remains, however, the only approved reperfusion therapy and when administered within three hours of symptom onset, results in a more than 30% increase in the number of patients returning to their premorbid level of functioning.
Novel neuroimaging methods and new strategies to open occluded vessels offer the potential to extend the therapeutic window for reperfusion of penumbral tissue although at present there is a paucity of evidence to support the expanded use of these treatment approaches. The challenge of the next decades will be to expand the availability of those acute stroke therapies with well-established evidence of efficacy to help offset the predicted rise in strokerelated morbidity and mortality expected over the next decades.
Secondary prevention of stroke
Introduction
With an estimated 20 million events occurring in the world each year, stroke is ranked as the sixth leading cause of global disease burden.97 Although declines in stroke incidence are emerging,98–100 aging and adverse lifestyle changes in populations will further intensify the impact of stroke and re-emphasize the importance of prevention and management strategies.101 Patients who experience a stroke or transient ischemic attack (TIA) are at substantial risk of a further serious vascular event (recurrent stroke, myocardial infarction or death from vascular cause). The risks of recurrent stroke are about 10% in the first week, 14% at one month, and 20% by three months after onset.102,103 Thereafter, the annual risks of recurrent stroke and myocardial infarction are around 5% and 2–3%, respectively.104 Given that 30–40% of patients with ischemic stroke have had a preceding TIA or minor stroke,105 and accumulating evidence indicates benefits from early assessment and management,106,107 TIAs serve as an important opportunity to intervene to prevent stroke.
This section reviews current evidence-based medical and surgical strategies used in reducing the risk of recurrent stroke and other vascular events after an ischemic stroke or TIA, or an intracerebral hemorrhage. Approaches to the prevention of subarachnoid hemorrhage or rarer forms of stroke, such as arterial dissection, are not considered.
Diagnosis of stroke and transient ischemic attack
Stroke is a clinical diagnosis, as outlined in a standard definition, of “ rapidly developing clinical signs of focal (or global) disturbance of cerebral function with symptoms lasting 24 hours or longer (or leading to death), with no apparent cause other than vascular origin ”.108 The term TIA refers to acute stroke symptoms (and signs) of the brain or eye that resolve within 24 hours.109 However, the advent of modern neuroimaging and use of thrombolysis for acute ischemic stroke has called into question the usefulness of the definition of TIA with a 24-hour time window for clinical decision making in the first few hours after the onset of symptoms.110 Even so, TIA remains a useful concept, both clinically and epidemiologically, as it does not rely on brain imaging and is a well-accepted diagnosis all over the world.
Pathologically, stroke generally occurs as a result of either ischemia or hemorrhage. Ischemic stroke, which accounts for about 80% of strokes in “ white ” populations, may occur via various mechanisms that include cardio-embolism (20–25% in Western population, e.g. secondary to atrial fibrillation (AF), valvular heart disease or patent foramen ovale), large-vessel atherosclerosis (approximately 50%, e.g. in situ
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


