Chapter 39 Sinus Node Dysfunction
In the early 1900s, Keith and Flack identified the sinus node as the region responsible for the activation of the heart.1 Laslett first suggested sinus node dysfunction as a cause of bradycardia in 1909, and during the 1950s and 1960s, Short, Ferrer, Lown, and others described the clinical spectrum of sinus node dysfunction commonly called sick sinus syndrome resting sinus bradycardia.2–5 Sinus node dysfunction can have multiple electrocardiographic manifestations, including sinus pauses, bradycardia-tachycardia syndrome, and inappropriate sinus node response to exercise (chronotropic incompetence).
Epidemiology
It can be difficult to differentiate sinus node dysfunction from physiological sinus bradycardia in a specific population. In a study of 50 young adult males, 24-hour ambulatory electrocardiographic monitoring revealed that 24% of the study group had transient heart rates less than 40 beats/min, pauses up to 1.7 seconds while awake, and 2.1-second pauses while asleep.6 Similarly, in a study of 50 young adult women, pauses from 1.6 to 1.9 seconds were observed.7 In older asymptomatic individuals, transient heart rates less than 40 beats/min and pauses of 1.5 to 2.0 seconds were observed in less than 2%.8 The decreased incidence of nocturnal bradycardia in this normal older adult population is probably caused by decrease in vagal tone that occurs with increasing age.
Sinus node dysfunction should be suspected when a patient describes symptoms of fatigue, syncope or presyncope, or exercise intolerance and is noted to have sinus bradycardia or pauses on the 12-lead electrocardiogram (ECG) or during Holter monitoring. In general, symptomatic sinus node dysfunction increases with age, with the incidence doubling between the fifth and sixth decades of life. In one study of approximately 9000 patients visiting a regional cardiac center in Belgium, Kulbertus et al estimated that the incidence of sinus node dysfunction is less than 5 per 3000 people older than 50 years of age.9 However, sinus node dysfunction is more commonly identified today because of an increased older adult population and increased physician awareness. In recent epidemiologic studies, sinus node dysfunction accounted for approximately 50% of the 300,000 new pacemakers implanted in the United States and 20% to 30% of the 900,000 new pacemakers implanted in Europe.10–12
Although more common in older adult patients, it is important to note that several specific younger patient groups can also have sinus node dysfunction. First, patients with congenital heart disease can have sinus node dysfunction. In 39 patients younger than 40 years of age who underwent pacemaker implantation for sinus node dysfunction at the Mayo Clinic, 64% had associated congenital heart disease.13 The most common condition was transposition of the great arteries corrected by a Mustard operation, since this procedure requires extensive atriotomies. Second, sinus node dysfunction is commonly observed after heart transplantation. Third, several familial forms of sinus node dysfunction have been identified and account for approximately 2% of patients who present with sinus node dysfunction.14–16 Bharati et al described a family with congenital absence of sinus rhythm. Several other investigators have described different forms of sinus node dysfunction that appeared to be genetically transmitted.14 Finally, sinus node dysfunction is observed in approximately 4% of patients after cardiac transplantation.17,18 Some, but not all, studies have found that a heart from a donor older than 40 years of age is associated with a higher incidence of sinus node dysfunction, necessitating permanent cardiac pacing.17,18 It appears that the incidence of permanent pacing after transplantation has decreased significantly with the widespread use of bicaval anastomoses rather than bi-atrial anastomoses.19
Anatomy
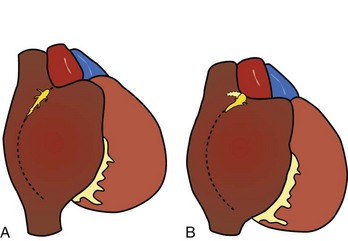
As described by Keith and Flack, the sinus node was shown to be lying in the terminal groove (sulcus terminalis), in the lateral part of the junction between the superior caval vein and the right atrium. This lateral position was endorsed by Koch and by most subsequent investigators.1,20–23 The horseshoe-shaped arrangement, with the node situated anteriorly and draped over the crest of the atrial appendage, as described by Hudson, is found in approximately 10% of hearts (Figure 39-1).24,25
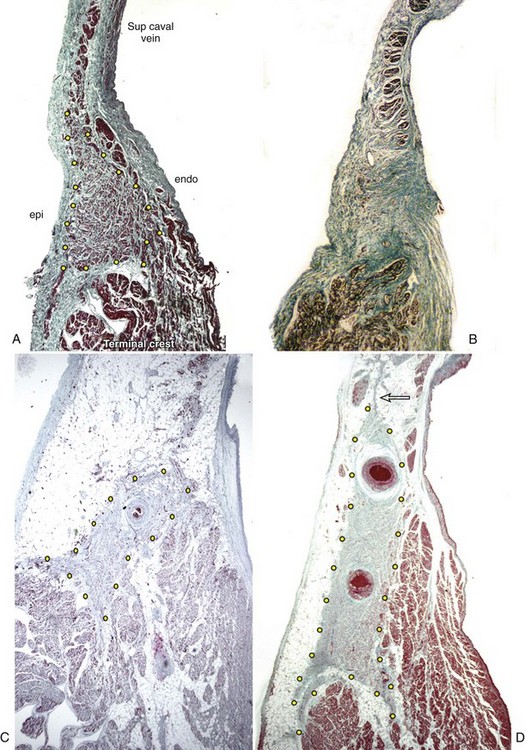
The shape of the node, most commonly, is like that of a tadpole, with the head section situated anterosuperiorly and the tapering tail extending for a variable distance inferiorly toward the entrance of the inferior vena cava.25,26 The fatty tissues of the terminal groove serve as the epicardial landmark, whereas the terminal crest (crista terminalis) in the anterolateral quadrant of the entrance of the superior vena cava is the endocardial landmark for the nodal head. In the adult heart, the nodal body is approximately 1 to 2 cm long, but the tail portion can extend considerably longer. In the subepicardium, the long axis of the node is parallel to the terminal groove, but the body and tail then gradually penetrate intramyocardially toward the subendocardium when traced inferiorly. Thus, while the nodal head is in the subepicardium of the terminal crest, the tail portion is close to the subendocardium.26
On histology, the node appears as a dense aggregation. The nodal cells are specialized myocytes that are histologically distinct from the ordinary myocardium of the atrial walls (Figure 39-2). They are small interlacing cells, less darkly stained, and with fewer myofibrils than in the neighboring ordinary myocytes. The specialized cells are associated with a fibrous tissue matrix. Ordinary myocardial cells are sometimes found scattered within the node. The nodal margins may be discrete, with fibrous separation from the atrial myocardium, or interdigitate through a short transitional zone. In the latter, tongues of nodal and transitional cells extend into the atrial myocardium. Prongs radiating from the nodal body are common. An anatomic study demonstrated 1 to 10 tongues of nodal tissue radiating mostly from the nodal body.26 The radiations penetrated the terminal crest in all directions, and some even extended into the muscular sleeve around the superior caval vein (see Figure 39-2). Another interesting observation from this study was the fragmentation of the tail portion of the node. In nearly two thirds of the nodes examined, the distal tail was traced to islands of nodal tissues among the fibro-fatty tissues and atrial myocytes in the subendocardium.
Innervation
The sinus node is the most densely innervated component of the cardiac conduction system.27,28 In the epicardium and in the environs of the node are collections of ganglion cells, which contribute vagal postganglionated nerve fibers to the node. The central part of the node is more densely populated with nerve fibers and bundles than are the peripheral regions.27
Arterial Supply
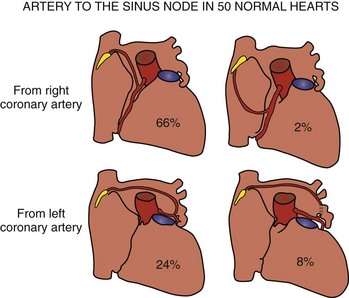
The artery supplying the node is a branch from the right coronary artery in 55% of hearts and from the left coronary artery in the remainder (Figure 39-3).29 When arising from the right coronary artery, it is usually the first atrial artery and ascends in the inter-atrial groove. Rarely, the artery arises near the acute margin from the lateral atrial artery to ascend the lateral wall of the right atrial appendage. The nodal artery is often a branch of the left anterior atrial artery when it arises from the left coronary system. In some hearts, it is a branch of the left lateral atrial artery. In both settings, it traverses the anterior wall of the left atrium and crosses the anterosuperior wall to reach the sinus node. The nodal artery approaches the node from an anterior direction in the majority of hearts but can also approach from a posterior direction or form an arterial circle around the cavoatrial junction.29 Typically, a prominent nodal artery passes centrally through the length of the nodal body and gives rise to small side branches along the way. In some hearts, the nodal artery branches as it approaches the node and several branches penetrate the nodal body. Occasionally, the artery passes along the side of the node and sends branches into the node. When the node is supplied by an arterial circle, the arteries enter the node at both ends.
Age-Related Changes
The sinus node displays marked histologic differences in infancy and in adulthood. In infants and in children, nodal cells predominate and the node appears relatively large compared with the bulk of the terminal crest. In the young individual, the node comprises nodal cells and fibrous tissue in nearly equal proportions.30 From about 30 years of age, fibrous tissue becomes more prominent, and the node appears smaller (see Figure 39-2).31 In the older adult, nodal cells decline, with increase in fibrosis. From 70 years onward, nodal cells may make up only 10% of the sinus node in patients who were in sinus rhythm up to death.31 However, as Shirashi and colleagues noted, the average volume of nodal cells in adults is 2.4 times more than in infants.32 This is mainly due to cellular hypertrophy. The same group found a 7.4 times increase in the volume of interstitial connective tissue in adult sinus nodes compared with that in infants. Another study using stains that are more specific for collagen tissue found an increase in collagen from infancy to adulthood, but the amount remains constant throughout adulthood.33 Contrary to general concepts, a detailed study has found that the loss of nodal cells is independent of the degree of arteriosclerosis.34 Fibrosis causing loss of continuity with the neighboring myocardium and increased fibrosis in the nodal approaches and internodal myocardium occur with increasing age. Using semi-quantitative assessment, Chow et al found dominance of sympathetic nerves initially in infancy, which gradually changed to co-dominance of sympathetic and parasympathetic nerves with increasing age.28
Sinus Node in Congenital Heart Malformations
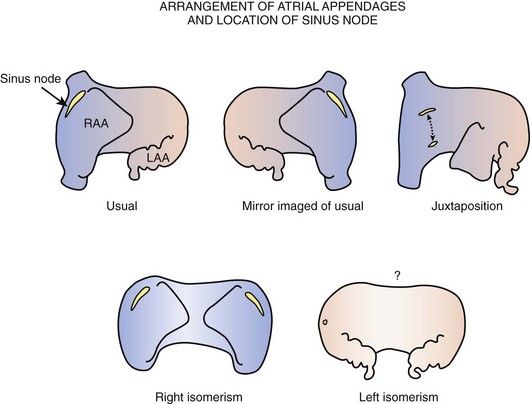
The majority of malformed hearts have the atrial chambers in their usual positions and the sinus node in its regular position. Abnormal positions of the sinus node have been found to occur in hearts with juxtaposition of the right appendage and in hearts with an atrial arrangement other than the usual type (Figure 39-4). Left juxtaposition exists when both atrial appendages lie to the left side of the arterial trunks rather than to either side at the base of the heart. In this malformation, the right appendage passes between the front of the atrial chambers and the great arteries. While the orifice of the superior vena cava remains in its usual position, the terminal crest is distorted, and the location of the sinus node is deviated. A study of six hearts with left juxtaposition showed a variable displacement of the node anteriorly (see Figure 39-4).35
Other malformations with abnormal positions of the sinus node are those that affect the arrangement of the atrial chambers. When atrial chambers are arranged in mirror-image fashion, the morphologically right atrium containing the sinus node is in the left-sided position. When bilateral right atrial appendages are present, as in right atrial isomerism, the sinus node is duplicated and is situated in both right and left positions.36,37 By contrast, when both atrial chambers are of left morphology, as in left atrial isomerism, the terminal crests are absent.37 Although, in this group, bilateral superior venae cavae can be present, the sinus node is not found in its usual position relative to either vena cava. When present, it is inferiorly displaced and smaller than usual. In some hearts, a remnant of specialized tissue is found in the posterior atrial wall near the atrioventricular (AV) junction, and in other hearts no nodal tissue can be identified.
Pathology
Many factors have been implicated in the etiology of sinus node dysfunction. Amyloid deposition within the sinus node and adjacent musculature is well recognized, as are anatomic findings of diffuse fibrosis or fibro-fatty replacement. Senile amyloidosis in older adults affects the atrial myocardium, including the approaches to the sinus node and the node itself. Marked loss of nodal cells associated with increased fibrosis or fatty changes of the atrial myocardium has been reported in sick sinus syndrome.33,38 Although usually seen in the older patient, these changes can also be seen in the young. Hypoplastic or atrophic sinus node may exist as a congenital anomaly that, with age-related loss of specialized cells, may progress to nodal dysfunction in later life or may be present in infancy (see Figure 39-2).39–41
Basic Electrophysiology of the Sinus Node
The structural and functional organization of the sinus node is quite complex, and significant differences are seen in the organization of the sinus node among species.42,43 The most extensive studies of the sinus node have been performed in rabbits. The rabbit node contains prototypical, small, structurally primitive pacemaker cells that are concentrated in the center; larger transitional latent pacemaker cells that are concentrated more peripherally; and intermingled, nonpacing atrial cells extending into the node from the atrial margins of the node in strands that are more prominent in the periphery. The cells within the node are relatively poorly coupled by gap junctions, and substantial interstitial tissue is interspersed among the fascicles of nodal cells.44 The resultant relatively poor intercellular communication slows the propagation of impulses from the central pacemaking regions toward the periphery of the node. In addition, the coupling of the transitional cells near the margins of the node with atrial myocardial cells is not a smooth continuum but consists of irregular junctions of interweaving strands of transitional cells and atrial cells extending into the interior of the node. Mapping of electrograms in and around the node has disclosed an apparent “multicentric” initiation of activation that likely represents irregular propagation to the atrial myocardium rather than simultaneous generation of impulses at separate sites. The node preferentially connects to the atrium in the superior aspects of the crista terminalis, and conduction block seems to exit the node in the direction of the atrial septum.
A dense representation of sympathetic and parasympathetic nerves and ganglia in the node ensures a sensitive autonomic responsiveness.45 With increasing vagal influence, the primary pacemaking site near the superior aspect of the node tends to migrate inferiorly, whereas an increasing adrenergic influence produces return to the primary dominant pacemaker sites in the superior region of the node. Detailed and sensitive analyses of P wave morphology and mapping of the sinus node indicate a dynamic shifting of pacemaker sites as well as changes in heart rate.
Action Potentials
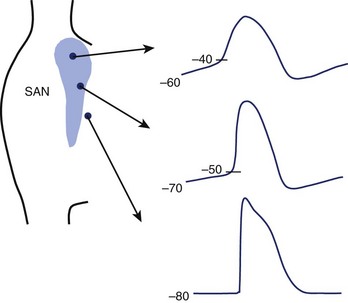
Small, primitive pacemaker cells in the interior of the node generate the dominant pacemaker potentials and show the least polarized maximum diastolic potentials (–60 to –40 mV), the most rapid rates of diastolic depolarization with smooth transition from end-diastole to the upstroke, and the slowest upstroke velocities (≈1 to 10 V/sec) (Figure 39-5).46 Latent pacemakers are concentrated more peripherally in the node. These cells are more polarized and show less rapid diastolic depolarization, a more abrupt transition from diastole to upstroke, and more rapid upstrokes. A continuous gradation of the properties of transitional cells—from the characteristics of primary pacemaker cells to the properties of highly polarized surrounding atrial cells with stable resting potentials close to –80 mV and upstroke velocities approaching 100 V/sec—can be seen. This gradation of properties is conditioned by cell coupling and electrotonic interaction as well as by the differing intrinsic properties of the myocytes within the node. Because of the electrotonic influence of atrial cells, the pacemaker capabilities of transitional cells are muted, whereas the more remotely connected interior cells are shielded from nonpacing atrial myocytes, allowing them to maintain pacemaker dominance.47 Separation of latent pacemaker cells near the atrial margins from the atrium and from the centrally located dominant pacemakers results in a faster intrinsic rate in latent pacemakers freed from the influence of nonpacemaking atrial cells. Cells in other parts of the atrium can also act as backup pacemakers, especially those in the inferior portions of the node near the coronary sinus. These pacemaker cells can respond appropriately to autonomic influence and, under abnormal conditions, can usurp control of the heart. The electrophysiological properties of these subsidiary pacemakers have not been as well characterized as those of the sinus node.
Recent studies indicate an influence of the fibroblasts within the node on pacemaker function and conduction.48 Inexcitable fibroblasts can couple with sinus node cells through gap junctions and depolarize the cells, reduce the rate of pacemaking, and impair conduction. Loss of sinus node myocytes associated with an increase in fibroblasts, collagen, and elastin has been implicated in sinus slowing and increased incidence of sinus node dysfunction and sinus node block with aging.
Detailed mapping by electrical and optical methods suggests that the sinus node is functionally connected to the atrial myocardium in discrete, relatively narrow sites, rather than diffusely along the margins of the node.49,50 At these sites, the propagating wavefront encounters a larger mass of the myocardium and source–sink mismatch that diminishes the safety factor for conduction. With certain conditions, including aging, that cause fibrosis or reduction of excitatory current, sinus node block may occur.
Currents
Sinus node pacemaker cells are relatively depolarized because of an absence or paucity of channels for the IK1 current.51,52 These channels are plentiful and open at negative membrane potentials in atrial and ventricular myocytes. They establish a dominance of K+ permeability in the resting state, thereby determining a resting potential approximating the K+
permeability in the resting state, thereby determining a resting potential approximating the K+ equilibrium potential (≈ –90 mV). The absence of these channels is most complete in the small, central pacemaker cells operating at diastolic potentials between –60 mV and –30 mV. In larger, more polarized transitional cells, IK1 may be present but reduced to varying degrees. The low upstroke velocities of these cells are related to a lack of operating sodium (Na+) channels, those channels that transmit the intense excitatory Na+ current in atrial and ventricular cells.52 The absence or paucity of this excitatory current is caused by a deficiency of the channels in individual myocytes and the depolarization of the myocytes during diastole to levels of membrane potential at which Na+ channels, if present, would be inactivated. The smallest cells may lack Na+ channels entirely. Larger transitional cells may contain Na+ channels and may operate at diastolic potentials at which Na+ channels can be activated to provide some excitatory Na+ current and more rapid upstrokes. The slow and diminutive upstrokes in the primary pacemaker cells are generated by the L-type Ca2+ current (ICaL), which serves as the trigger for release of Ca2+ by the sarcoplasmic reticulum and therefore the trigger for contraction in all cardiac cells but is the primary excitatory current in depolarized sinus nodal cells. This current is slower and far less intense than the Na+ current, accounting for the poor upstrokes and slow conduction within the node. The currents producing diastolic depolarization, which is the fundamental pacemaker potential, comprise a multitude of candidates, but no uniform consensus about this exists at present.53–57 The “funny” current If, encoded by HCN4, is a nonspecific cation current that is mainly an inward Na+ current activating relatively slowly at negative membrane potentials in the range of diastolic potentials.58 It becomes more intense at more negative membrane potentials. This current is well expressed in sinus nodal cells, responds appropriately to adrenergic and cholinergic stimulation, and thus is a plausible candidate to be an important pacemaker current. However, some studies have shown activation at more negative levels than the diastolic potentials of the primary pacemakers (below –60 mV) and a greater representation in peripheral latent pacemakers.
equilibrium potential (≈ –90 mV). The absence of these channels is most complete in the small, central pacemaker cells operating at diastolic potentials between –60 mV and –30 mV. In larger, more polarized transitional cells, IK1 may be present but reduced to varying degrees. The low upstroke velocities of these cells are related to a lack of operating sodium (Na+) channels, those channels that transmit the intense excitatory Na+ current in atrial and ventricular cells.52 The absence or paucity of this excitatory current is caused by a deficiency of the channels in individual myocytes and the depolarization of the myocytes during diastole to levels of membrane potential at which Na+ channels, if present, would be inactivated. The smallest cells may lack Na+ channels entirely. Larger transitional cells may contain Na+ channels and may operate at diastolic potentials at which Na+ channels can be activated to provide some excitatory Na+ current and more rapid upstrokes. The slow and diminutive upstrokes in the primary pacemaker cells are generated by the L-type Ca2+ current (ICaL), which serves as the trigger for release of Ca2+ by the sarcoplasmic reticulum and therefore the trigger for contraction in all cardiac cells but is the primary excitatory current in depolarized sinus nodal cells. This current is slower and far less intense than the Na+ current, accounting for the poor upstrokes and slow conduction within the node. The currents producing diastolic depolarization, which is the fundamental pacemaker potential, comprise a multitude of candidates, but no uniform consensus about this exists at present.53–57 The “funny” current If, encoded by HCN4, is a nonspecific cation current that is mainly an inward Na+ current activating relatively slowly at negative membrane potentials in the range of diastolic potentials.58 It becomes more intense at more negative membrane potentials. This current is well expressed in sinus nodal cells, responds appropriately to adrenergic and cholinergic stimulation, and thus is a plausible candidate to be an important pacemaker current. However, some studies have shown activation at more negative levels than the diastolic potentials of the primary pacemakers (below –60 mV) and a greater representation in peripheral latent pacemakers.
The If current is enhanced in the diastolic potential range of sinus node cells by activation of adenylate cyclase, for example, with adrenergic (sympathetic) stimulation, increasing cyclic adenosine monophosphate (cAMP) in the cytosol. Binding of cAMP to the channel increases the probability of the channel opening in the voltage range of –40 mV to –100 mV. Cholinergic (vagal) stimulation reduces cAMP, the If current, and the rate of pacemaking. Downregulation of HCN4 and If with prolonged atrial tachycardia may account for the impairment of sinus node function in atrial fibrillation and tachycardia.59,60
The importance of If in pacemaking in human sinus node had been validated by recent discoveries. Mutations in HCN4 impair sinus node pacemaking.56,61 Selective blockers of If have been demonstrated to slow sinus rate in human subjects.58 If has been demonstrated in sinus node cells from excised human sinus node.62
In the absence of IK1, the delayed rectifier K+ currents IKr and IKs, which are activated during the action potential, can play a role in the attainment of the maximum diastolic potential by providing the K+ permeability that would bring the transmembrane potential close to the K+ equilibrium potential at the end of the action potential when they are fully activated. As these currents deactivate in diastole, the membrane potential would drift toward the more positive equilibrium potentials of other major ions such a Na+, Ca2+, and Cl–. It has been argued that one or another of the delayed rectifier currents is the major pacemaker current.54 However, although IKs has an appropriate autonomic sensitivity, IKr does not. IKs is not prominent in the sinus nodes of all species.
It has recently been proposed that inward Na+ /Ca2+ exchange current (NCX) activated by spontaneous release of Ca2+ from sarcoplasmic reticulum is a major depolarizing current in late diastole in sinus node cells. This postulated “internal Ca2+ clock” is driven by high levels of phosphorylation of Ca2+-cycling proteins, modulated by adrenergic and cholinergic stimulation.57
Etiology
Sinus node dysfunction results from various conditions that have in common the capability to depress automaticity in and electrical conduction from the sinus node and perinodal and atrial tissues. These conditions can be intrinsic, resulting from structural damage to the sinus node, or extrinsic, caused by medications or systemic illnesses. The clinical and electrocardiographic manifestation of sinus node dysfunction is inappropriate sinus or atrial bradycardia.63–66 Some patients may also experience episodes of supraventricular tachycardias (tachycardia-bradycardia syndrome). Because the clinical manifestations of sinus node dysfunction can mimic normal physiological conditions (bradycardia) or can be caused by diseases that do not affect the sinus node (supraventricular tachycardias), the assessment and management of patients with suspected sinus node dysfunction can be challenging.67–69
Intrinsic Causes of Sinus Node Dysfunction
Intrinsic sinus node dysfunction is usually caused by degenerative processes involving the sinus node and the sinus node area. The syndrome is usually acquired but can rarely be familial.8 Sinus node dysfunction is present when inappropriate sinus bradycardia, pauses in sinus rhythm (sinus arrest), sinus node block, or a combination of these exist.70 The degenerative process and associated fibrosis may also involve the AV node and intraventricular conduction system; as many as 17% of patients with sick sinus syndrome have evidence of AV block and bundle branch block.71
Natural History
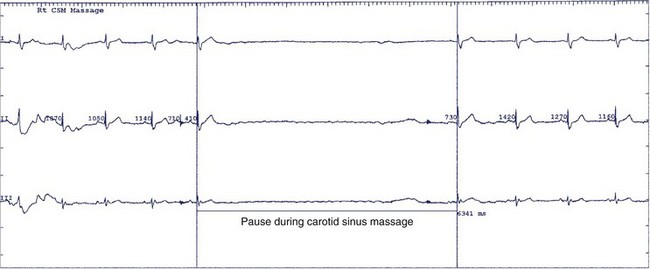
The natural history of sick sinus syndrome is one of spontaneous clinical improvement alternating with periods of clinical deterioration. Patients generally seek medical attention when they are symptomatic from bradycardia. In the majority of cases, the heart rate spontaneously increases, and symptoms diminish.72–74 However, the clinical course is not predictable. Even patients with more severe symptoms such as syncope may remain free of symptom recurrence for years, and slightly more than half do not experience another syncopal episode over a 4-year follow-up.74 No clear explanation exists for this erratic course of the syndrome. The autonomic nervous system may play an important role in the genesis of symptoms, especially in the trigger of syncope.74 The prevalence of abnormal responses to carotid sinus massage (pauses exceeding 3 seconds) and tilt table testing is significantly higher in patients who experience syncope than in those who do not, highlighting the contribution of abnormal neural reflexes in the pathophysiology (Figure 39-6).74,75
Although symptoms are common in patients with sick sinus syndrome, survival is usually not affected, even in patients who develop syncope.76 Death related directly to dysfunction of the sinus node occurs in less than 2% of patients over 6 to 7 years of follow-up.77,78 However, patients with sick sinus syndrome often have comorbid conditions that can shorten their lifespan. Coronary atherosclerosis is the most prevalent among these conditions, although myocardial ischemia and infarction, congestive heart failure, and advanced age are also common.79,80 In one report, patients with sick sinus syndrome had a 4% to 5% excess annual mortality in the first 5 years of follow-up compared with an age-matched and sex-matched population.81 However, the mortality in patients without other coexisting diseases at the time of diagnosis of sinus node dysfunction did not differ significantly from that observed in controls. Thus, although medical intervention and permanent cardiac pacing may be required to improve symptoms, no data exist supporting the claim that these management strategies improve survival.
Clinical Manifestations
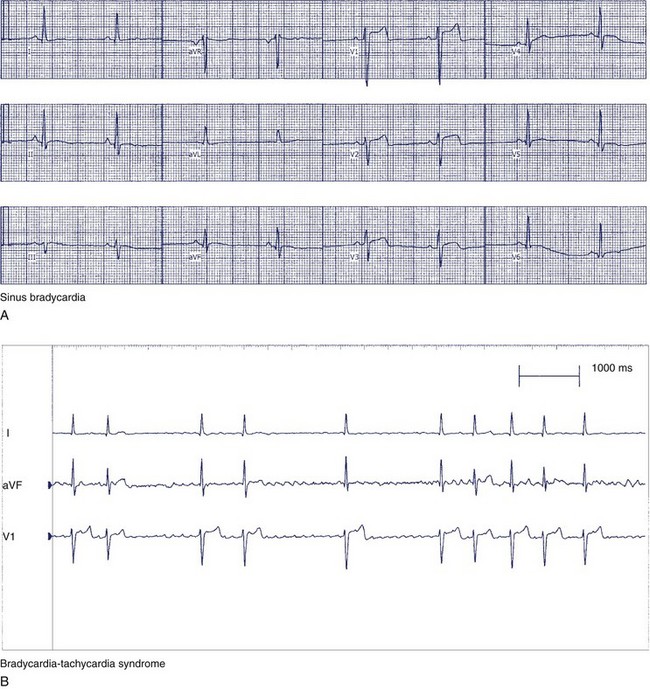
The clinical manifestations of sick sinus syndrome are caused by both bradycardia and tachycardia. In bradycardia-tachycardia syndrome, patients experience paroxysmal episodes of supraventricular tachycardia, which can be atrial tachycardia, atrial flutter or fibrillation, or re-entry tachycardia (Figure 39-7). More than one type of tachycardia may occur in the same patient. Episodes of rapid heart rate can lead to palpitations, angina, and syncope. Conversely, slowing of the heart rate without a compensatory increase in stroke volume leads to a reduction in cardiac output, which results in fatigue, weakness, lightheadedness, and dizziness.
Failure of the heart rate to increase appropriately with exercise (chronotropic incompetence) is a manifestation of sinus node dysfunction. Proposed definitions for chronotropic incompetence include failure to reach 85% of age-predicted maximum heart rate at peak exercise, failure to achieve a heart rate above 100 beats/min, or a maximal heart rate during exercise greater than two standard deviations below that of a control population.82–84 In patients with chronotropic incompetence, symptoms may be present only during activity.
In about 17% of patients with sick sinus syndrome, overt congestive heart failure may develop, which may be related or contributed to by the slow heart rate, loss of the atrial contribution to left ventricular filling in patients who develop atrial fibrillation, or loss of AV synchrony in patients with implanted ventricular pacing systems.73
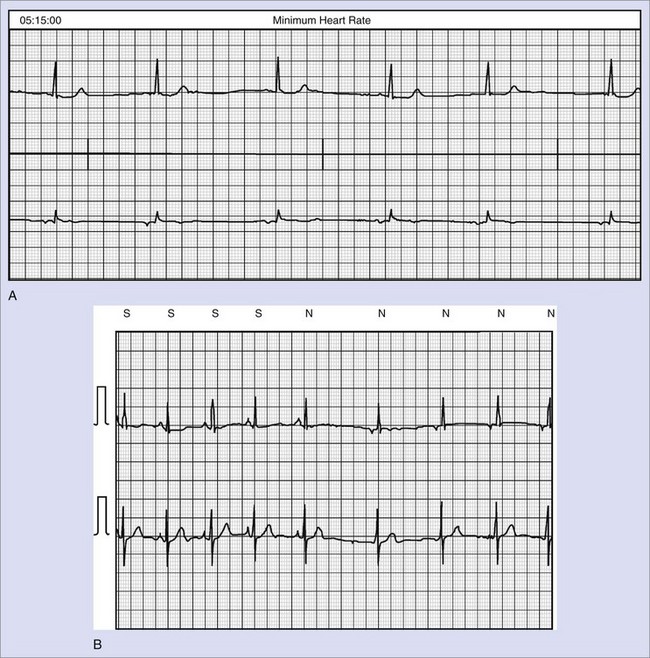
Syncope or severe presyncope is one of the classical manifestations of sick sinus syndrome and occurs in about 25% of patients. In one prospective trial, the actuarial rates of syncope were 16% and 31% after 1 and 4 years of follow-up, respectively.73,74 Syncope is usually caused by excessive slowing of or transient pauses in sinus rhythm or by sinus exit block with an inadequate escape rhythm (Figure 39-8). In patients with bradycardia-tachycardia syndrome, overdrive suppression of the sinus node may occur when the tachycardia terminates, resulting in prolonged pauses and syncope. Use of antiarrhythmic medications in these patients, while successful in controlling the tachycardia, frequently leads to worsening of the bradycardia episodes with exacerbation of symptoms, and permanent cardiac pacing is required for rate support.
About one third to one half of patients with sick sinus syndrome experience episodes of supraventricular tachycardia.73,78 Published reports indicate that chronic atrial fibrillation occurs in about 11% of cases after a mean follow-up of 19 months, increases to 16% at 5 years, and to 28% at 10 years.73,74,84 Variables that have been associated with the development of chronic atrial fibrillation are age; left ventricular end-diastolic diameter; presence of valvular heart disease; and ventricular, rather than physiological, atrial or dual-chamber, pacing.84–86 Chronic atrial fibrillation is also more common in patients with tachycardia-bradycardia syndrome who have had paroxysmal atrial fibrillation. When atrial fibrillation develops, many patients previously symptomatic from bradyarrhythmia experience a substantial improvement, likely due to the increase in ventricular rate.87
Thromboembolic events, which occur in 3% to 9% of patients, may be a manifestation of sick sinus syndrome.73,74 In patients with bradycardia-tachycardia syndrome, the incidence increases to 24%.56 Ventricular pacing (as opposed to atrial or dual-chamber pacing) and the presence of pre-existing cerebrovascular disease are also associated with a higher thromboembolic event rate during follow-up.84–86 High-risk patients should be carefully identified and placed on long-term anticoagulation therapy. However, not all cerebrovascular accidents in these patients are caused by embolic events. Older adults with atherosclerotic cerebrovascular disease may have transient ischemic attacks or frank cerebral infarction if bradyarrhythmia or tachyarrhythmia is associated with a fall in cardiac output and reduction in cerebrovascular perfusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree