Vascular access complications (VACs) remain one of the biggest challenges when performing transcatheter aortic valve implantation (TAVI). This study aimed to investigate the short- and medium-term safety and efficacy of the Viabahn endoprosthesis (Gore, Flagstaff, AZ) when used to treat TAVI-induced vascular injury. Over a 40-month period, 354 patients underwent true percutaneous transfemoral (TF)-TAVI using a CoreValve and Prostar-XL closure system; this was our study population. A VAC leading to acute intervention occurred in 72 patients (20.3%) – of these, 18 were managed by balloon angioplasty, 48 were treated by Viabahn stenting (technical success rate 98%), and 6 needed surgical intervention. Overall, this approach resulted in a major VAC rate of 3.1% (n = 11) in our study cohort. Length of hospitalization and 30-day mortality rates were comparable in patients with a VAC treated by Viabahn stenting versus patients without vascular complications. Two patients (4.5%) presented with new-onset claudication; one of them had the stent implanted covering the deep femoral artery (DFA). At medium-term follow-up (median 372 days; range 55 to 978 days) duplex ultrasound showed 100% patency of the Viabahn endoprostheses with no signs of stent fracture or in-stent stenosis/occlusion. In conclusion, the use of self-expanding covered stents is safe and effective in case of TF-TAVI–induced vascular injury, with good short- and medium-term outcomes. Importantly, coverage of the DFA should be avoided. If confirmed by long-term (>5 years) follow-up studies, this strategy for treating TAVI-induced VAC may be used routinely in high-risk patients.
Transcatheter aortic valve implantation (TAVI) has emerged as a new therapeutic option for patients with severe symptomatic aortic stenosis, who are ineligible or at high risk for conventional surgical aortic valve replacement. The transfemoral (TF) route is the first choice in most centers performing TAVI. As an alternative to surgical cutdown to the femoral artery, a true percutaneous TAVI procedure has the potential to shorten procedure duration, reduce the risk of wound infection, and keep patient immobilization and discomfort and length of hospitalization to a minimum. A main reason not to choose a true percutaneous approach for TAVI is the concern for major vascular access complications (VACs), which are a major cause of morbidity and mortality. The use of self-expanding covered stents as treatment option for vascular injuries after TAVI has been described. However, to our knowledge, a systematic and longer term evaluation of this approach is not available. Therefore, the aim of this study was to investigate the short- and medium-term safety and efficacy of the Viabahn endoprosthesis (Gore, Flagstaff, AZ) when used to treat TAVI-induced vascular injury.
Methods
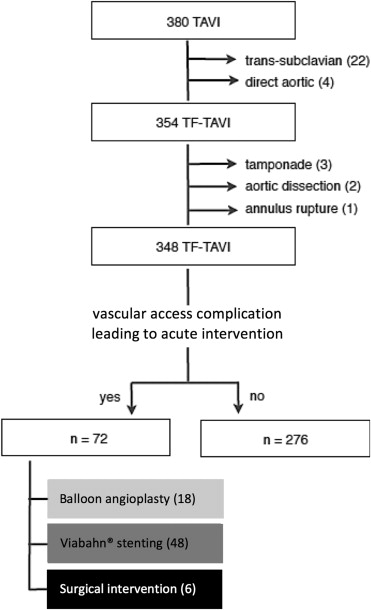
Over a 40-month period, a total of 380 patients underwent TAVI in our center using the self-expanding CoreValve (Medtronic, Minneapolis, MN). All patients had a computed tomographic (CT) angiography and/or iliofemoral angiography to assess the size (>6 mm), tortuosity, and degree of calcification of the iliofemoral axis. In total, 354 patients were treated by TF approach; this was our study population. In accordance with the institutions’ policies, every patient gave written informed consent for TAVI and the use of anonymous data for research in accordance with the Ethical Committee (Region Hovedstaden) review board approval.
All TF-TAVI procedures were performed under general anesthesia using percutaneous access and conventional suture-mediated closure using the Prostar-XL vascular closure system (Abbott Vascular, Chicago, IL). Vascular access was obtained by a contralateral femoral artery puncture and introduction of a 6Fr sheath. Fluoroscopic and contrast-guided ipsilateral access was supported by an internal mammary artery catheter from the contralateral side through crossover technique above the aortic bifurcation. In this way, a high puncture of the ipsilateral common femoral artery (CFA) above the femoral artery bifurcation was facilitated, and a 6Fr sheath was introduced. After skin incision (10 mm) and careful blunt dissection of the subcutaneous tissue, the Prostar-XL device was advanced over a 0.035-in guidewire into the vessel, and the sutures were deployed according to the instructions for use. Next, the Prostar-XL delivery system was removed over a J-tip guidewire and replaced by a 12Fr sheath to further dilate the femoral arteriotomy. After exchange to an 18Fr sheath over an Amplatz Super Stiff guidewire, CoreValve implantation was performed. At the end of the procedure, the preloaded sutures were tied, and angiographic control of the access vessel was performed through a contralateral internal mammary artery catheter to evaluate vascular injury and hemostasis.
In the event of a vascular access site injury, a predefined protocol was followed. Minor vascular injury at the puncture site was treated with either external compression or balloon angioplasty for 3 to 5 minutes using a Tyshak balloon (NuMED, Hopkinton, NY) introduced through a 6Fr to 8Fr Arrow sheath (Teleflex, Ireland) from the contralateral side (and repeated for 2 to 3 times in some cases). If this strategy failed or when major extravasation was apparent on angiographic control, a 50-mm-long Viabahn endoprosthesis ( Figure 1 ) was inserted covering the vessel lesion, aiming to place the distal part of the stent proximal to the bifurcation of the CFA into superficial femoral artery (SFA) and deep femoral artery (DFA). If endovascular repair was impossible (e.g., because a wire could not pass the lesion), considered not suitable by the operator (too extensive vascular injury), or unsuccessful in achieving vessel patency, surgical vessel repair was performed.

All end points were defined according to the Valve Academic Research Consortium 2 (VARC-2) recommendations, including bleeding, vascular complications, and acute kidney injury. In March 2014, the information contained in our database was re-evaluated to conform to the definitions proposed by the VARC-2. Other end points included procedural variables, units of blood transfusion, length of hospitalization at intensive care unit (ICU) and total length of hospitalization, and 30-day mortality.
All patients who underwent TAVI and received a Viabahn endoprosthesis were invited to participate at the follow-up study. One patient was lost to follow-up, and 1 other patient died before the time of follow-up. The following parameters were assessed: (1) symptoms of claudication according to the Rutherford classification; (2) femoral and pedal (arteria dorsalis pedis [ADP] and arteria tibialis posterior [ATP]) pulses; (3) ankle-brachial index (ABI; normal range 0.9 to 1.3); and (4) a color duplex ultrasound (US) of the external iliac artery (EIA), CFA, proximal DFA, and proximal SFA was performed to ensure stent visualization and perform flow measurements proximal, within, and distal of the stent and also around any plaque, if present. If a patient presented with worsening of symptoms, a significant change in ABI, or a reduced flow in the proximal DFA or SFA, more extensive imaging (CT angio or full SFA and popliteal US) was performed.
All statistical analyses were performed with SPSS version 20 (IBM SPSS Inc., Chicago, Illinois). Categorical variables are reported as absolute values and percentages. Continuous variables are presented as means ± SD or medians and interquartile range (IQR). Comparisons between groups were performed using the chi-square test (categorical variables) and the Student t test (continuous variables). Statistical significance was defined as p <0.05.
Results
Access-related complications (VAC) leading to acute intervention occurred in 72 patients (20.3%) of the study population and were more frequently observed in female patients or patients with diabetes mellitus, a high body mass index, or a high surgical risk score (p <0.05, Table 1 ). Of these, 18 were treated with balloon angioplasty, 48 with a Viabahn endoprosthesis, and 6 needed surgical vessel repair ( Figure 2 ). Viabahn endoprostheses were used for different types of VAC: stenosis (n = 3), dissection (n = 4), incomplete vascular closure due to closure device failure (n = 36), and rupture/perforation (n = 5; Figures 3 and 4 ). The use of a Viabahn endoprosthesis was the primary choice in 38 patients, whereas in 10 other patients, stenting was preceded by balloon angioplasty with unsatisfactory result.
| Variable | Acute VAC Intervention | p value | |
|---|---|---|---|
| No | Yes | ||
| (n = 282) | (n = 72) | ||
| Age (years) | 79 ± 11 | 80 ± 6 | 0.331 |
| Female | 115 (40.8%) | 40 (55.6%) | 0.034 ∗ |
| Arterial hypertension | 153 (54.3%) | 47 (65.3%) | 0.121 |
| Hypercholesterolemia | 120 (42.6%) | 39 (54.2%) | 0.102 |
| Diabetes mellitus | 45 (16.0%) | 20 (27.8%) | 0.037 ∗ |
| Body mass index (kg/m 2 ) | 25.7 (23.5-28.7) | 26.9 (24.0-32.1) | 0.032 ∗ |
| Previous myocardial infarction | 21 (7.4%) | 9 (12.5%) | 0.256 |
| Previous PCI | 69 (24.5%) | 24 (33.3%) | 0.169 |
| Previous CABG | 70 (24.8%) | 22 (30.6%) | 0.401 |
| Atrial fibrillation | 93 (33.0%) | 25 (34.7%) | 0.889 |
| Peripheral artery disease | 26 (9.2%) | 13 (18.1%) | 0.054 |
| Chronic kidney disease | 106 (37.6%) | 30 (41.7%) | 0.618 |
| Chronic lung disease | 44 (15.6%) | 12 (16.7%) | 0.968 |
| Angina pectoris | 101 (35.8%) | 30 (41.7%) | 0.435 |
| Syncope | 38 (13.5%) | 7 (9.7%) | 0.512 |
| NYHA III-IV | 197 (69.9%) | 45 (62.5%) | 0.291 |
| Log EUROScore | 12.7 (8.1-21.3) | 16.5 (12.3-23.3) | 0.048 ∗ |
| LVEF (%) | 50 ± 13 | 52 ± 11 | 0.126 |
| Peak gradient (mmHg) | 68 ± 26 | 74 ± 26 | 0.117 |
| Mean gradient (mmHg) | 40 ± 17 | 44 ± 17 | 0.130 |
| Aortic valve area (cm 2 ) | 0.68 ± 0.16 | 0.68 ± 0.16 | 0.922 |
| Aortic regurgitation ≥ grade 2 | 10 (3.5%) | 0 (0.0%) | 0.222 |
| Valve-in-valve | 17 (6.1%) | 4 (5.6%) | 0.862 |
| Predilatation | 248 (87.9%) | 69 (95.8%) | 0.082 |
| Valve size (mm) | 28.3 ± 1.7 | 28.1 ± 1.8 | 0.323 |
| Postdilatation | 27 (10.5%) | 9 (12.9%) | 0.732 |
The total procedure length was prolonged with 9 to 11 minutes in case of endovascular repair, whereas in case of surgical intervention, the procedure was extended for >85 minutes. In contrast, almost a double radiation dose was used in case of endovascular repair, whereas extra radiation was hardly used in case of surgical repair ( Table 2 ).
| Variable | No acute intervention | Balloon angioplasty | Viabahn ® stenting | Surgical intervention |
|---|---|---|---|---|
| (n = 276) | (n = 18) | (n = 48) | (n = 6) | |
| Procedural variables | ||||
| Contrast volume (mL) | 104 ± 47 | 132 ± 37 | 136 ± 49 | 118 ± 71 |
| Radiation (Gy x cm 2 ) | 102 ± 96 | 180 ± 162 | 191 ± 173 | 123 ± 91 |
| Total procedure length (min) | 69 ± 27 | 78 ± 37 | 80 ± 28 | 157 ± 107 |
| Bleeding | ||||
| Major | 2 (0.7%) | 1 (5.6%) | 3 (6.3%) | 1 (16.7%) |
| Life-threatening | 1 (0.4%) | 0 (0.0%) | 1 (2.1%) | 2 (33.3%) |
| Blood transfusion (units) | ||||
| ≥ 1 | 44 (15.9%) | 4 (22.2%) | 11 (22.9%) | 5 (83.3%) |
| ≥ 4 | 1 (0.4%) | 0 (0.0%) | 1 (2.1%) | 2 (33.3%) |
| Vascular complication | ||||
| Minor | 5 (1.8%) | 17 (94.4%) | 43 (89.6%) | 1 (16.6%) |
| Major | 0 (0.0%) | 1 (5.6%) | 5 (10.4%) | 5 (83.3%) |
| Acute kidney injury | ||||
| ≥ stage 1 | 7 (2.5%) | 1 (5.6%) | 2 (4.2%) | 2 (33.3%) |
| ≥ stage 2 | 3 (1.1%) | 0 (0.0%) | 1 (2.1%) | 1 (16.6%) |
| Length of hospitalization (days) | ||||
| Intensive care unit | 1.3 ± 0.6 | 1.5 ± 0.7 | 1.4 ± 0.7 | 3.8 ± 1.4 |
| Total length | 7.6 ± 3.8 | 8.9 ± 5.3 | 7.8 ± 4.3 | 12.5 ± 6.1 |
| 30-day mortality | 2 (0.7%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


