Fig. 12.1
Growth and development of the lung are mechanically coupled to that of the thorax. Adapted from [5]
Innate Potential for Compensatory Lung Growth
Lower vertebrates such as newts and salamanders are well known for their ability to regrow a completely new and fully functional limb or tail to replace one that has been lost [35]. While mammals cannot regenerate an entire lung , under appropriate stimulation, mature mammalian lungs retain the ability to add new gas exchange structures including the intra-acinar airways (respiratory bronchioles, alveolar ducts, and alveolar sacs) and alveolar septa (cells, matrix, fibers, and capillaries). When some lung units are destroyed by disease or surgery, the remaining units expand under the negative intrathoracic pressure leading to unfolding of alveolar surfaces. Simultaneously, pulmonary perfusion to these units increases at a given cardiac output, leading to microvascular recruitment and augmentation of lung diffusing capacity (Fig. 12.2). As loss of lung units continues, physical stress and deformation of tissue and microvasculature exceed a critical threshold, at which point structural growth is stimulated. The newly generated cells, matrix, fibers, and capillaries undergo remodeling or architectural adjustment of the alveolar septa, fibroelastic scaffold , and broncho-vasculature, ultimately leading to balanced increases in all major acinar components. Remodeling is a critical step that redistributes mechanical stress, maximizes air–tissue and tissue–blood interface areas, minimizes tissue–blood barrier resistance to diffusion, and optimizes ventilation-to-perfusion and perfusion-to-diffusion matching. The end result is a larger gas exchanger with a higher lung diffusing capacity above that expected from the initially remaining fraction of lung units under a given set of conditions, thus achieving the goal of functional compensation (Fig. 12.2). Conversely, generation of structural components that fail to directly or indirectly support the functional goal is not compensatory growth.
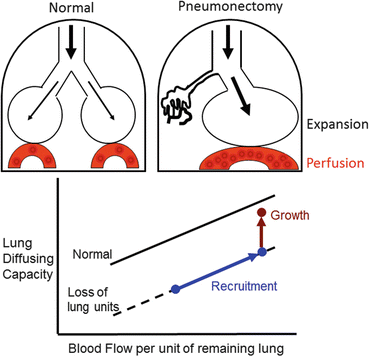

Fig. 12.2
Two types of complex mechanical signals following loss of lung units—expansion of the remaining parenchyma, and increased perfusion to the remaining microvasculature. These signals recruit alveolar-capillary volumes and surface areas, leading to a higher lung diffusing capacity as blood flow per unit of remaining lung increases (blue line and symbols). When mechanical signals exceed a threshold intensity, structural growth is stimulated with a further increase in lung diffusing capacity at any given pulmonary blood flow above that expected per unit of initially remaining lung
Pneumonectomy Model of Compensatory Lung Growth
Owing to the invasive procedures needed to obtain human lung tissue, animal models have been indispensible in the investigation of lung growth. From a mechanistic standpoint, major surgical lung resection (e.g., pneumonectomy ), a widely investigated model that consistently demonstrates robust compensatory growth of the remaining lung in all species examined including the mouse, rat, rabbit, ferret, dog [36–45], and indirectly in human subjects with functional loss of one lung [16, 17, 46–49]. Major lung resection mimics the loss of gas exchange units caused by obliterative disease, e.g., pulmonary fibrosis . However, unlike most models of lung injury, pneumonectomy removes a known and highly reproducible fraction of functioning lung units; the resulting signals and responses of the remaining lung units are readily quantifiable. Over more than a century, this model has been extensively characterized at physiological, structural, cellular, and molecular levels and utilized to study the sources and magnitudes of adaptation, the determinants of structural growth and remodeling as well as eventual functional outcome. Cumulative results strongly implicate mechanical stimuli in the re-initiation, modulation, and limitation of lung growth.
Pneumonectomy was first performed in dogs and rabbits in 1881, reviewed by [50]. By the 1920s, it was known that animals function quite well with one expanded remaining lung that fills the entire thoracic cavity [51–53], and surgeons began to perform major lung resection in patients. Studies in the 1950s showed that dogs [54] and patients [55] tolerate staged removal of up to 70 % of lung mass. Subsequently, rodents were used extensively for characterizing the cellular and molecular basis of accelerated post-pneumonectomy lung growth while the canine model remains useful for relating structure to function, defining the sources and limits of adaptation, and evaluating translational interventions.
Mechanical Signal–Response Relationships
In all species, the magnitude of post-pneumonectomy compensatory lung growth correlates inversely with age and maturation stage. Thus, compared to young animals compensatory growth in adults requires a higher threshold for initiation, a longer time course of adaptation [49] with early cell proliferation and progressive scaffold remodeling [56] that only partially normalizes structure–function [57, 58]. Critical pathways that are normally activated during lung development are further upregulated; nondevelopmental pathways may also be recruited [59]. The magnitude and distribution of compensatory growth vary with the fraction of lung units removed. Following canine left pneumonectomy (~42% resection), only the most caudal (infracardiac) remaining lobe exhibits significant growth of alveolar tissue and surface area; compensation is derived predominantly from alveolar-capillary recruitment and parenchyma remodeling. Following right pneumonectomy (58 % resection), alveolar growth intensifies in magnitude and uniformity in all lobes; compensatory gains in structure and function exceed that following 42 % resection [60]. Following 65–70 % lung resection, alveolar growth is still vigorous but with diminishing gains in structure and function [57] (Fig. 12.3). This pattern signifies a threshold, an optimal range, and an upper limit of the mechanical signal–response relationship following increasing loss of lung units.
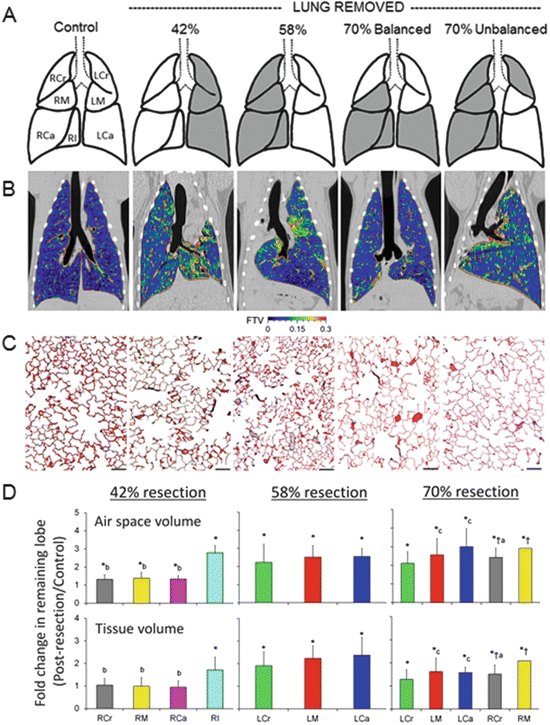

Fig. 12.3
Structural response to increasing loss of lung units by pneumonectomy —42, 58, and 70 % balanced and 70 % unbalanced resection. (a) The adult canine lobes removed are shaded in gray. (b) Coronal HRCT images are shown at the level of the carina. The color scale indicates in vivo fractional tissue volume (FTV) of lung parenchyma. (c) Representative micrographs of the distal lung. Bar = 200 μm. (d) Average fold changes in airspace volume (upper) and extravascular alveolar tissue volume (lower) in individual remaining lobes following different degrees of lung resection, expressed as ratios with respect to the same lobe in normal control animals. The lobes were fixed by tracheal instillation at a constant airway pressure. Left: 42 % resection. Middle: 58 % resection. Right: 70 % resection (balanced and unbalanced groups combined). Right lobes: RCr right cranial (gray), RM right middle (yellow), RCa right caudal (magenta), RI right infracardiac (aqua). Left lobes: LCr left cranial (green), LM left middle (red), LCa left caudal (blue). Mean ± SD. P ≤ 0.05: *vs. control (1.0); †vs. 42 %; avs. RM, bvs. RI, cvs. LCr. Adapted from [57]
Following 42 % resection, lobar expansion initially unfolds the remaining alveolar septa with little tissue stress in most lobes except the infracardiac lobe, which lies between the heart and the diaphragm, preferentially expands across the midline, increases nearly twofold increase in tissue volume and partially reconstitutes the cardiac fossa. Type-2 pneumocyte volume increases first, before that of other septal cell types, suggesting its sensitivity to mechanical stress and consistent with its role as a resident progenitor cell [61]. Following 58 % lung resection, expansion and stress in all lobes exceed a critical threshold, leading to uniform growth initiation, stress relief, and greater functional enhancement per unit of initially remaining lung compared to 42 % resection. The initial rapid post-pneumonectomy airspace expansion is followed by further gradual expansion in the subsequent days as intrathoracic air and fluid are resorbed. An increase in tissue permeability recruits fluid, cells (especially monocytes and macrophages ), chemokines, and cytokines to the remaining lung leading to a disproportionate (3.6-fold) increase in the volume of septal interstitial cells and matrix (excluding collagen). With time, the gain in interstitial volume wanes, and all major septal components—epithelium , interstitium, endothelium, and capillary blood—increase to about two (1.5–2.5) fold of that in the control lung (sham pneumonectomy) (Fig. 12.4). Signals related to airspace expansion account for approximately half of the observed post-pneumonectomy structure–function compensation (Fig. 12.5); the remainder is attributable to perfusion-related stimuli although nonmechanical factors may also play a role [62, 63]. As resection increases from 58 to 65–70 %, the diminishing gains in growth and compensation suggest the counter-balancing effects of excessive mechanical stress that may heighten cellular oxidative stress and damage tissue integrity at the expense of growth-related activities. An excessive increase in pulmonary vascular resistance may also impair tissue adaptive response. However, new capillaries (evidenced by an increase in double alveolar-capillary profiles) continue to form with increasing loss of lung units without reaching a plateau up to 70 % resection, suggesting the existence of separate perfusion-related stimuli for microvascular growth [57].
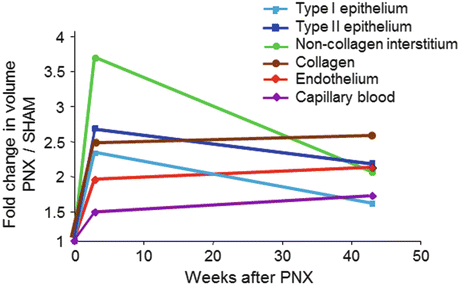
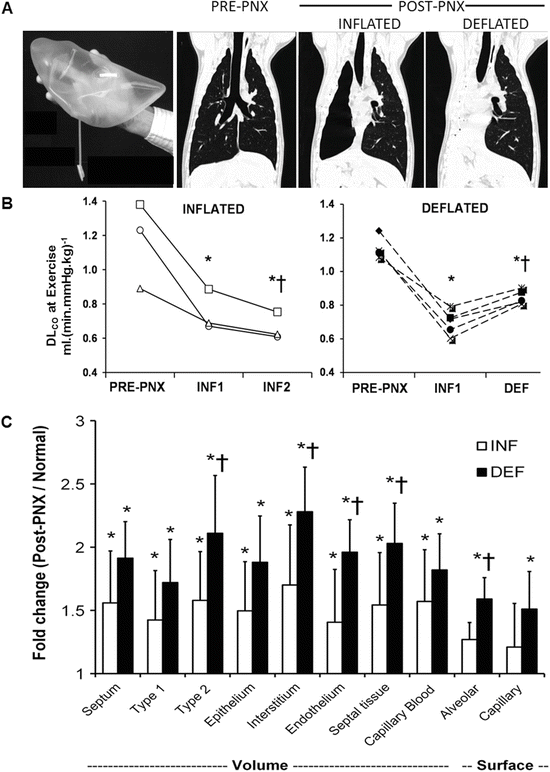

Fig. 12.4
Increases in the compartmental volumes of the alveolar septa in young dogs (2.5 months age) 3 weeks and 1 year following 58 % resection by right pneumonectomy (PNX), expressed as ratios to the corresponding mean values in litter- and gender-matched control animals following sham pneumonectomy. Note the early disproportionate increase in the volume of non-collagen septal interstitium

Fig. 12.5
Contribution of lung expansion to post-pneumonectomy (PNX) growth and compensation. Upper panels: Adult canine right lung was replaced with custom-shaped inflated silicone prosthesis following right PNX to minimize expansion of the remaining lobes. High-resolution computed tomography was performed at a transpulmonary pressure of 30 cmH2O pre- and 4 months post-PNX (with inflated prosthesis, INF), and then 4 months following acute deflation of the prosthesis (~8 months after surgery, DEF). Control animals underwent right PNX with continuously inflated prosthesis and also studied at 4 and 8 months post-PNX (INF1 and INF2, respectively). Middle: Lung diffusing capacity (DLCO) was measured during exercise and expressed at a constant cardiac output of 400 mL (min kg)−1 in individual animals pre-PNX and at the two time-points post-PNX. P ≤ 0.05: *vs. pre-PNX and †vs. INF1 by repeated measures ANOVA. Lower: Fold increase in volumes and surface areas of alveolar septal components measured 1 year following right PNX in animals with inflated or deflated prosthesis, expressed as ratios to the average values in normal controls. Mean ± SD. P ≤ 0.05: *vs. control (1.0), †vs. INF. From [62, 63]
Quantifying Regional Mechanical Stimuli
Using in vivo high-resolution computed tomography, nonrigid image registration and deformation analysis, we visualized regional parenchyma deformation (displacement, strain, and shear) and compliance, and quantified growth of the functional parenchyma, which includes alveolar tissue as well as microvascular blood [56, 64]. Results demonstrate markedly nonuniform regional displacement, deformation, and growth with inflation (Fig. 12.6), with a prolonged course of adaptation marked by initial parenchyma growth, progressive tissue relaxation, stress relief, and gradual functional improvement over many months [56, 58, 64] (Fig. 12.7). A protracted temporal course of adaptation is probably fundamentally important to ensure the coordination and optimization of homeostatic pathways at micro and macro scales and to minimize architectural distortion. Intuitively, one might expect nonuniform spatial stimuli-response to weaken the average or the whole lung compensation at a given stimulus intensity. On the other hand, nonuniformity also prolongs the “window of susceptibility” during which mechanical stimuli remain active at least in some parts of the lung, thereby rendering the overall adaptive response amenable to intervention.
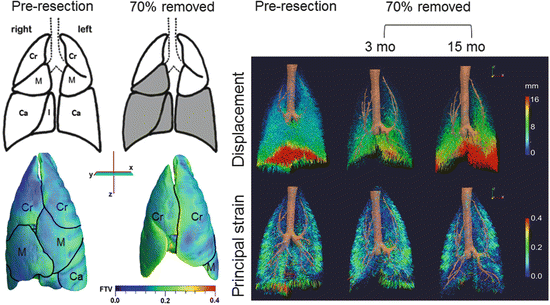
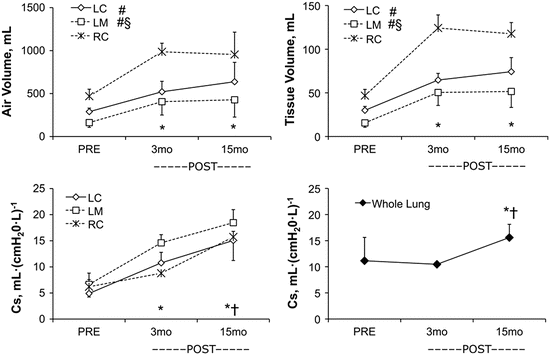

Fig. 12.6
Parenchyma deformation quantified by high-resolution computed tomography. Left upper panels: Diagrams of normal adult canine lobes and those remaining following balanced 70 % resection—the removed lobes are shaded in gray. Left lower panels: Representative three-dimensional reconstruction shows marked expansion of the remaining three lobes (demarcated by black lines) mainly in a caudal direction and around the mediastinum. The color map shows heterogeneous subpleural fractional tissue volume (FTV). Lobes: Cr cranial, M middle, Ca caudal. Right panels: Three-dimensional vector field maps of parenchyma displacement (upper) and principal strain (lower) during inflation (from 15 to 30 cmH2O transpulmonary pressure) before and 3 and 15 months after 70 % resection illustrate temporal and spatial mechanical heterogeneity during compensation. At 3 months post-compared to pre-resection, displacement magnitude is reduced and principal strain increases nonuniformly in the enlarged remaining lobes compared to the same lobe pre-resection. At 15 months compared to 3 months post-resection, displacement increases markedly particularly in caudal regions, and regional strain is nonuniformly reduced [88, 89]

Fig. 12.7
Growth and remodeling phases in the remaining lobes 3 and 15 months following 65–70 % lung resection assessed by high-resolution computed tomography. Upper panels: Lobar air and tissue volumes increase significantly from PRE to 3 months POST-resection then remain stable between 3 and 15 months. Lower left panel: Specific compliance (Cs) of the remaining three lobes continue to increase from 3 to 15 months POST-resection. Lower right panel: Whole lung Cs (representing seven lobes PRE and three lobes POST) did not change from PRE to 3 months POST then increased between 3 and 15 months POST. Mean ± SD. Lobes: RC right cranial, LC left cranial, LM left middle. Repeated measures ANOVA with post hoc analysis by Fisher’s Protected Least Significant Difference. Comparison with respect to time: P < 0.05, *vs. PRE, †vs. 3 months POST in all remaining lobes. Comparison among lobes: P < 0.05, #vs. RC, §vs. LC. From [56]
Structural Basis of Compensatory Growth and Remodeling
Postnatal compensatory lung growth is thought to be mediated mainly via recruitment and activation of resident progenitor cells in response to local signals while circulating bone marrow-derived cells or vascular progenitor cells play no role or at best a minor role [65–67]. Alveolar septation is a four-dimensional event involving spatio-temporal coordination of hundreds of genes and thousands of mediators. The bulk of gas exchange occurs through the “thin side” of septa, which contains minimal tissue with high barrier conductance. Structural support of septa is concentrated in the “thick side,” which contains most of the cells, matrix, and fibers. As the elastin fibers coursing throughout the “thick side” of alveolar wall are pulled under tension, they may lift and “fold” an existing alveolar capillary, create a tissue pillar that transects the capillary lumen leading to a “double-capillary” profile typical of the developing lung . Tension on elastin fibers may also lift tissue and capillary constituents out of their two-dimensional plane to create a new septum. Subsequently, cell proliferation, matrix deposition, and fiber rearrangement separate the “double-capillary” and remodel it into two single capillaries typical of the mature lung. This process, termed “intussusception” [68], could generate a new septum or expand an existing alveolar tissue-capillary sheet depending on the balance of forces (Fig. 12.8).
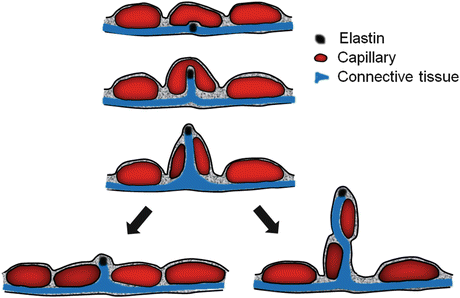

Fig. 12.8
Diagram of intussusceptive capillary growth and formation of a new alveolar septum. See text for explanation
At the level of acinar airways, alveolar ducts contain smooth muscle and other contractile elements in their incomplete walls, which form the entrance rings to alveolar sacs [3]. Following pneumonectomy , alveolar ducts increase in number and volume [69]. Branching of the most distal alveolar ducts could add one more airway generation to double the total alveolar tissue volume and surface areas. This is a likely mechanism because tissue deformation is disproportionally larger in subpleural than central lung regions [56] corresponding to a similar gradient of cell proliferation and growth factor expression [12, 13]. Alternatively, alveolar sacs may bud from the terminal bronchiole that forms the entrance to an acinus, transforming the terminal bronchiole into another generation of respiratory bronchiole. It remains unclear which, or perhaps both, of these mechanisms are operative during compensatory growth.
At the level of the conducting broncho-vasculature, further branching is not possible. A different type of growth occurs post-pneumonectomy , namely traction-related elongation and dilatation, which must also involve the generation of additional tissue components. Elongation along each airway or vascular generation increases, while dilatation mitigates, the increase in flow resistance. As luminal flow resistance is directly proportional to the length and inversely proportional to the fourth power of the radius (Poisuille’s Law), only a small increase in radius is needed to offset the effects of lengthening. Bronchovascular adaptation is less vigorous or complete than alveolar adaptation, leading to dissociated compensation or “dysanaptic” lung growth [38, 70], where pulmonary limitation upon exercise is primarily limited by the persistently and disproportionately elevated airway and pulmonary vascular resistances and not by the reduction in lung diffusing capacity [71].
Species Differences in Lung Growth and Compensation
Large mammalian lungs differ from rodent lungs in several aspects of anatomy, development , and maturation that impact adaptation. Bronchovascular stratification is simplified in rodents. Respiratory bronchioles are few and short in the rabbit, guinea pig, hamster, gerbil, rat, and mice [72–74]. In human and canine lungs, acinar airways bifurcate through several generations of respiratory bronchioles and alveolar ducts to end in alveolar sacs. Extensive stratification allows modulation of ventilation–perfusion distributions and the penetration, deposition, and clearance of inhaled particles. Relative to rodent lungs, the highly stratified large lungs need stronger connective tissue and fibers for support, which in turn requires a more rigid rib cage to maintain stability but not too rigid as to restrict truncal flexibility in locomotion. Stratification also creates a longer mean acinar path length, which requires more smooth muscle and contractile elements to fine-tune ventilation–perfusion–diffusion matching among the regions and at different stratified levels. In the vasculature, murine tracheobronchial capacitance vessels may not penetrate into intrapulmonary airways [75, 76], and perturbation of pulmonary blood flow readily stimulates angiogenesis of chest wall and pleural vessels [77]. In large animals, extensive bronchial and pulmonary precapillary anastomoses [78] provide ample collateral circulatory reserves, which may minimize the need for new vessel formation. These structural differences could explain the ease with which the lungs of small animals may be stimulated to regrow while the lungs of large animals rely heavily on nonstructural adaptive mechanisms; structural growth is stimulated only when the nonstructural reserves have been exhausted.
Translational Challenges in Lung Growth Induction
Mechanical signals are the only stimuli known to re-initiate adult lung growth de novo [5, 79]. While the innate growth potential is retained in adult lungs, the incomplete regrowth response is in need of ways of augmentation. This issue is critical because current therapy for chronic lung disease is non-curative except for lung transplantation, which is burdened by problems of donor availability and complications. The key unanswered questions include the following: (1) How to maximally realize the innate ability for regrowth and compensation of functioning lung units in obliterative disease and reduce the need for lung transplantation or replacement? (2) How to re-initiate lung growth in the absence of adequate mechanical stimuli such as in emphysema? (3) How to optimize alveolar cellular repopulation and capillary regrowth in bioengineered lungs?
The pneumonectomy model has proved useful for examining the integrated network interactions elicited by mechanical stimulation and for evaluating the efficacy of therapeutic approaches. Post-pneumonectomy exposure to ambient hypoxia [80, 81] and the administration of exogenous growth promoter molecules, proteins, and DNA [82, 83] have been shown to modestly enhance selective aspects of lung growth in rodents, although none has been shown to improve function. We examined this question in adult canines by administering oral all trans-retinoic acid (RA) for 3 months post-pneumonectomy. In the absence of active endogenous compensatory growth (left pneumonectomy), RA supplementation has no significant effect [83]. In the presence of endogenous growth activities (right pneumonectomy), RA supplementation modestly enhances growth of type-1 pneumocytes, interstitium, endothelium, and capillary blood volume as well as alveolar and capillary surface areas compared to placebo treatment [82, 84]. The expected increase in double-capillary profiles is also accentuated consistent with RA-enhanced capillary formation and remodeling. On the other hand, RA supplementation minimally stimulates the growth of alveolar type-2 pneumocytes, causes thickening of the alveolar-capillary basement membrane and the septa, and fails to significantly enhance long-term whole lung function [82, 84, 85]. These results suggest that pharmacological intervention could augment mechanically induced lung growth but is insufficient for re-initiating growth in the absence of sufficient mechanical signals. What factors prevent the translation of exogenously enhanced structural growth into functional gain? The answer remains speculative although a few possibilities should be considered:
1.
Most studies tested a single exogenous growth promoter at a pharmacological dose over a short duration. This approach is conceptually inadequate because endogenous compensatory lung growth involves sustained, low-intensity, balanced amplification of numerous mechano-transduction pathways to gradually enlarge all parts of the gas exchanger, minimize distortion, and optimize outcome. Intense unbalanced stimulation could distort the blood gas barrier and neutralize the intended benefit. Broad stimulation of multiple key pathways at a low dose over a longer duration may be more effective in enhancing overall growth and function.
2.




Scaffold remodeling continues long after cessation of accelerated cellular proliferation. As mechanical stress is redistributed and the local mechano-transduction micro-milieu shifts, different molecular pathways may be favored at different time points in the course of response. Selective administration of exogenous growth promoters that sequentially target each specific phase of the natural response may prove more effective.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


