Chapter 72 Risk Stratification for Sudden Cardiac Death
Sudden cardiac death (SCD) is defined as natural death from cardiac causes that occurs within 1 hour from the onset of symptoms. If unwitnessed, patients should have been seen alive within 24 hours preceding their death. Pre-existing heart disease may be known; nevertheless, SCD frequently affects persons with no previously recognized cardiovascular disorder.1 However, as documented by autopsy studies of 270 cases of SCD in individuals with no previously known heart disease, pathologic examination revealed structural abnormalities in 95%.2 The real incidence of SCD is difficult to establish, mainly because of the different definitions of SCD and the sources of data used in published reports. On the basis of the latest meta-analysis of reports published between 1980 and 2007, the estimated annual incidence of SCD in the United States varies from 180,000 to more 450,000 annually.3 SCDs account for approximately 50% of all cardiac deaths, and this proportion has remained constant despite the overall decline in cardiovascular death during the last decades.4,5 Recent decades have shown a steady decline in the total number of cardiac arrests, which is attributed mainly to a decrease in out-of-hospital ventricular fibrillation (VF). Nevertheless, survival after a cardiac arrest remains as low as 5%.5–8
Mechanisms of Sudden Cardiac Death
SCD may result from ventricular tachycardia (VT), VF, bradyarrhythmias, or pulseless electrical activity. Before the widespread use of implantable cardioverter-defibrillators (ICDs), data on the final arrhythmia leading to cardiac arrest could only be obtained from studies using Holter recordings, hospital telemetry, or both.9–13 The largest study to date on SCD episodes occurring while the patients were wearing a Holter monitor was published in 1989 by Bayes de Luna et al.12 Among 157 subjects, VT degenerating to VF was observed in 62% of cases, torsades de pointes in 13%, and primary VF in 8%. Only 17% of patients died from bradyarrhythmia. Conversely, the proportion of patients with bradyarrhythmia or electromechanical dissociation as a mechanism of SCD, as documented by Liu et al, may be higher in heart failure patients.13 This observation was later supported by the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) trial, which showed that in heart failure patients, the mechanism of death depends on hemodynamic impairment. Patients with New York Heart Association (NYHA) heart failure class II to III are more likely die suddenly, whereas heart failure progression is responsible for the majority of those in NYHA class IV.14
Recent data based on electrocardiograms (ECGs), from implantable loop recorders in patients with prior myocardial infarction (MI) from the Cardiac Arrhythmias and Risk Stratification after Acute Myocardial Infarction (CARISMA) study confirmed that ventricular tachyarrhythmias were associated with SCDs, whereas bradyarrhythmia and electromechanical dissociation were predominantly observed in non-SCD and noncardiac deaths.15,16 The CARISMA study enrolled 312 patients with a recent acute MI (AMI) and left ventricular ejection fraction (LVEF) less than 40%. Terminal arrhythmias were recognized from loop recorders implanted 5 to 21 days after an AMI. During 2 years of follow-up, 26 patients died: 9 from SCDs, 10 from non-SCD cardiac causes, and 7 from noncardiac causes. Among those with SCD, VF was observed in 6 cases and bradyarrhythmia only in 1 (in 2 cases no rhythm was available for analysis). In contrast to previous Holter-based reports, initiation of VF (visible in 5 cases) was not preceded by VT in any of the cases. These data confirm that primary VF is the main mechanism of death in patients with prior MI and left ventricular dysfunction.
Multi-factorial Etiology of Sudden Cardiac Death: Impact on Risk Stratification
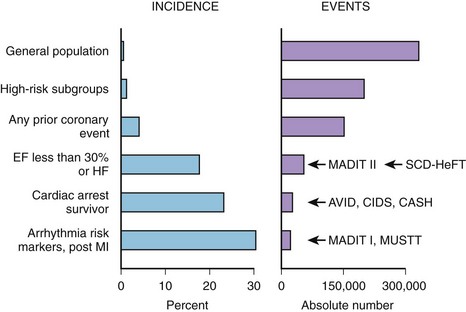
Coronary artery disease (CAD) remains the most common condition associated with SCD and is responsible for approximately 80% of cases.1 The highest risk of SCD is attributed to cardiac arrest survivors, and these patients are qualified for ICD implantation for secondary prevention of death. Indications for primary prevention of SCD are based on the underlying heart disease that was proven to increase the risk of arrhythmic events. Patients with prior MI and significantly depressed LVEF and those with heart failure or ventricular arrhythmia present with the highest incidence of SCD events.17,18 Nevertheless, as indicated by Myerburg et al in a review of the population impact of emerging ICD trials, the highest incidence of SCD is observed in survivors of out-of-hospital cardiac death and high-risk post-infarction subgroups, but the highest absolute number of SCD events occurs in large subgroups of patients at a somewhat lower risk, including patients with left ventricular dysfunction, congestive heart failure (CHF), or any prior coronary event (Figure 72-1).
The etiology of SCD is multi-factorial and involves a complex interplay among several elements, including genetic predisposition, cardiac condition, comorbidities, and environmental factors. Such a complex underlying etiology makes risk prediction of “unexpected” SCD events extremely complicated. The mechanisms of SCD include VT, VF, bradycardia, asystole, and pulseless electrical activity. Risk prediction is therefore focused mainly on predicting tachyarrhythmias, which can be prevented by ICD therapy. Life-threatening arrhythmias are preceded by a chain of events encompassing the complex interplay among the substrate, triggers, and modulators. Factors known to modulate and trigger arrhythmias include autonomic nervous system imbalance, transient ischemia, metabolic and electrolyte imbalance, transient volume overload of ventricles, and proarrhythmic action of drugs. Malignant arrhythmias are induced and sustained by a series of triggering and modulating events acting on the vulnerable myocardium.19
Role of Noninvasive Electrocardiology in Risk Stratification
Even though SCD is defined as being unexpected and frequently occurs as a first clinical event in previously asymptomatic persons, attempts have been made to identify risk markers indicating increased predisposition for arrhythmic events.20
Depressed EF that persists for years has been recognized as a major determinant of increased mortality rate, so the assessment of LVEF remains the gold standard in identifying patients who benefit from ICD therapy.17,21–24 The highest risk of death and SCD is attributed to a subgroup of patients with LVEF less than 30%. In comparison with earlier studies, recent series have suggested that the curve relating death to EF has “shifted to the left,” implying that for a given degree of left ventricular dysfunction, the increase in mortality rate is somewhat less than previously reported. Furthermore, the main drawback of EF in risk stratification lies in its low sensitivity and specificity.25,26
Noninvasive ECG parameters are believed to reflect the myocardial substrate as well as triggers and modulators potentially contributing to life-threatening arrhythmia risk. Therefore, the combination of ECG markers with low EF may potentially enhance risk stratification. Several ECG markers have been introduced into SCD risk stratification over the past few decades. Most of them have been associated with all-cause mortality and cardiac death. Nevertheless, the association with SCD is unclear, and the results of studies are conflicting.20,27–29 Detailed descriptions of specific risk stratification methods are available in other chapters in this text that detail these methods.
Electrocardiographic techniques include surface ECG, signal-averaged ECG (SAECG), ambulatory Holter monitoring, exercise tests, and other techniques such as baroreceptor sensitivity assessment. Surface ECG provides data on the underlying rhythm, resting heart rate, QRS duration, conduction abnormalities, QRS fragmentation, Q-T interval duration, and T-wave morphology or specific changes suggestive of primary electrical diseases.29–31
SAECG reveals the presence of late potentials reflecting heterogeneity of conduction in the myocardium. The presence of late potentials, prolonged filtered QRS duration, or both in the SAECG of patients with normal QRS duration on standard ECG indicates an increased risk of cardiac events. A broad QRS complex is associated with an increased risk of death, and patients with such conduction disturbances do not benefit from SAECG analyses.30,31
Long-term Holter monitoring allows evaluation of heart rate, ventricular ectopy, heart rate variability (HRV), heart rate turbulence (HRT), and dynamicity of repolarization.20,27–29 Ambulatory Holter monitoring is the most comprehensive tool for identifying and quantifying factors that may contribute to the mechanism of SCD (Figure 72-2). The effects of the autonomic nervous system on the heart could be evaluated by quantifying HRV, illustrating the relationship between the parasympathetic and sympathetic components of this system. HRT complements HRV analysis by providing insight into a baroreflex sensitivity component of central regulation of the cardiovascular system.28 Abnormalities of the central regulation of the heart are unlikely to cause SCD without an altered myocardial substrate and additional factors increasing the vulnerability of the myocardium to VT. The vulnerability of the myocardium may be expressed as impaired repolarization dynamicity, increased frequency and complexity of ventricular arrhythmias, and transient ischemic ST-T changes.
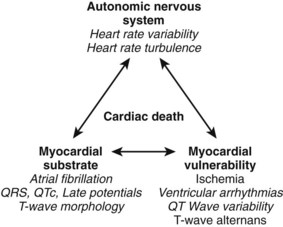
FIGURE 72-2 Factors contributing to cardiac death and respective Holter-derived electrocardiogram parameters.
(From Zareba W, Moss AJ: Noninvasive risk stratification in postinfarction patients with severe left ventricular dysfunction and methodology of the MUSIC II noninvasive electrocardiology substudy, J Electrocardiol 36[Suppl]:101–108, 2003.)
Exercise tests go beyond evaluating ST-T changes and provide additional information on the risk of SCD based on heart rate behavior and ventricular ectopy during the recovery phase.32 Microvolt T-wave alternans (TWA), considered to be a reflection of repolarization abnormalities, was originally determined during an exercise test, but numerous recent data support the equal value of TWA assessed by long-term monitoring.33,34
Invasive Electrophysiology Study
Testing the inducibility of VT in patients with prior MI has been a standard procedure for a number of years for identifying high-risk patients prone to SCD. Two primary prevention clinical trials—the Multicenter Automatic Defibrillator Implantation Trial (MADIT) and the Multicenter Unsustained Tachycardia Trial (MUSTT)—which enrolled patients with prior MI and depressed LVEF who presented with nonsustained VT (NSVT) and inducibility of ventricular tachyarrhythmias during invasive electrophysiology study (EPS), demonstrated that such a risk stratification algorithm was able to select a subset of patients with prior MI and a very high mortality risk.21,35 Nevertheless, secondary analysis from MUSTT revealed that despite significant differences in outcome between inducible patients enrolled in the trial and noninducible patients enrolled in a EPS registry, inducibility was found to be of limited use because the 5-year mortality rate in inducible patients was 48% compared with 44% in noninducible patients.36 Subsequently, MADIT II showed that additional risk stratifiers (including EPS) are not necessary when EF is severely depressed.22 In fact, in more than 80% of patients randomized to the ICD arm of MADIT II, invasive EPS, in an attempt to induce tachyarrhythmias, was performed at the time of ICD placement. Inducibility of ventricular arrhythmias, observed in 40% of studied patients, was not effective in identifying patients with cardiac events defined as VT, VF, or death.37 Therefore, MUSTT and MADIT II subanalyses suggested that in patients with substantially depressed left ventricular function, inducibility at EPS should not be considered a useful predictor of outcome. However, inducibility may have much better predictive value in patients with prior MI and an LVEF greater than 30% or less than 35%. Such a hypothesis was confirmed in a study by Cappato et al, who investigated the usefulness of inducibility in 285 survivors of cardiac arrest enrolled in the Cardiac Arrest Study Hamburg (CASH) trial and found that inducibility at EPS was predictive for SCD in patients with LVEF greater than 35% (hazard ratio [HR], 3.0; P = .006), whereas it was not useful in patients with lower LVEF (HR, 1.1; P = .81).38
Inducibility in patients with nonischemic cardiomyopathy has not been considered useful for predicting increased risk of death. However, data from the Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) substudy, which evaluated ICD-based inducibility of VT or VF, found that inducibility of either VT or VF was associated with an increased likelihood of subsequent ICD therapy for VT or VF.39
Bedside Risk Stratification Models
In risk stratification for SCD or cardiac death, attempts are frequently made to use sophisticated noninvasive or invasive methods reflecting ECG-based electrical myocardial involvement or autonomous nervous system regulation of the cardiovascular system. However, evaluating the risk of death could also be accomplished by using clinical parameters readily available to physicians when assessing patients in hospitals and in outpatient clinics. Retrospective data analyses from the ICD trials MADIT II, MUSTT, and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) yielded risk stratification models based on such clinical variables.40–42
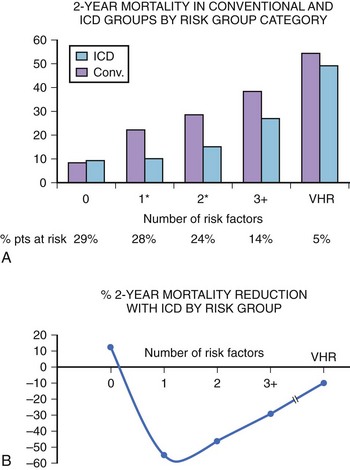
The simplest bedside risk stratification model was proposed by the MADIT II investigators, who evaluated five clinical factors: (1) NYHA class higher than class II, (2) age greater than 70 years, (3) blood urea nitrogen level greater than 26 mg/dL, (4) QRS duration greater than 0.12 seconds, and (5) atrial fibrillation (AF). Crude mortality rates in the conventional group were 8% and 28% in patients with zero or one or more risk factors, respectively, and 43% in very high-risk patients, who were defined by a blood urea nitrogen level of 50 mg/dL or more, serum creatinine level of 2.5 mg/dL or more, or both (Figure 72-3). Defibrillator therapy was associated with a 49% reduction in the risk of death (P < .001) among patients with one or more risk factors (n = 786), whereas no ICD benefit was identified in patients with no risk factors (n = 345; HR, 0.96; P = .91) and in very high-risk patients (n = 60; HR, 1.00; P > .99). This model could easily be adopted and could significantly improve low utilization of ICDs for primary prevention of death and SCD in patients with low EF and prior MI.
| Inducible VT | 17 |
| History of CHF | 19 |
| Patient enrolled as inpatient | 17 |
| EF ≤20% (for EF values between 20% and 40%, add one point for each EF percentage point >40) | 20 |
| EF = 40% | 0 |
| NSVT not discovered within 10 days after bypass grafting | 17 |
| IVCD or LBBB | 10 |
The SCD-HeFT investigators adopted the Seattle Heart Failure Model for predicting mortality and ICD benefit in their study population. The model included age, gender, systolic blood pressure, ischemic origin, NYHA class, LVEF, angiotensin-converting enzyme inhibitor use, angiotensin receptor blocker use, β-blocker use, statin use, furosemide equivalent daily dose in milligrams per kilogram, serum sodium, digoxin use, carvedilol use, and creatinine. This relatively complex model yielded scores categorizing patients in quintile subgroups. ICD treatment decreased the relative risk of SCD by 88% in the lowest risk group versus 24% in the highest risk group (P = .009 for interaction) and decreased the relative risk of total mortality by 54% in the lowest risk group versus no benefit (2%) in the highest risk group (P = .014 for interaction).41
The Muerte Subita en Insufficiencia Cardiaca (MUSIC) study was designed to evaluate risk predictors for SCD in patients with mild to moderate CHF (NYHA class II to III). In a cohort of 992 ambulatory patients, a risk score based on 10 variables (prior atherosclerotic vascular event, left atrial size >26 mm/m2, LVEF ≤35%, AF, LBBB or IVCD, NSVT, frequent ventricular premature beats [VPBs], estimated glomerular filtration rate <60 mL/min/1.73 m2, hyponatremia ≤138 mEq/L, N-terminal pro–B-type natriuretic peptide >1000 ng/L, and positive troponin) identified ambulatory patients with CHF at high risk of total mortality and SCD during follow-up.42
Risk Stratification in Patients with Prior Myocardial Infarction and Ischemic Cardiomyopathy
CAD remains the condition most commonly associated with SCD. Patients with prior AMI are at significant risk of SCD during the first few months after the index event. However, data from patients with AMI and heart failure or depressed EF indicate that during first 3 months, SCD is most frequently caused by recurrent MI or rupture of the ventricle.43 The risk of SCD from tachyarrhythmia tends to be relatively low during the first few years but increases in later years after MI when remote remodeling of the myocardium takes place. Yap et al, on the basis of a combined analysis of five multi-center trials that enrolled patients with prior MI and LVEF less than 40% or frequent VPBs, documented that in this high-risk subgroup the risk of arrhythmic death is higher than that of nonarrhythmic death for up to 2 years of post-infarction follow-up, with the highest risk during the first 6 months after MI.44 With time, the risk of arrhythmias decreases, but the risk of death from heart failure persists. Nevertheless, the absolute risk of death from any cause was the highest in the first 6 months after MI and decreased with time. Interestingly, nonarrhythmic death occurred more among females after 6 months. The risk of arrhythmic death was also higher in younger patients.44
Historically, detecting and quantifying Holter-recorded ventricular arrhythmias was the first ECG-based approach to determine the risk of patients with prior MI and to implement antiarrhythmic therapy.45 Ventricular arrhythmias are associated with acute ischemia caused by partial or total occlusion of coronary arteries. In patients with ischemic cardiomyopathy, monomorphic VT may result from re-entry mechanisms in the scarred areas of the myocardium. An association exists between the increased frequency and the complexity of ventricular arrhythmias with cardiac death and SCD. In a classic study by Bigger et al, the occurrence of 10 or more VPBs per hour was associated with an increased mortality rate.46 Subsequent results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2) trial confirmed that frequent VPBs (>10 per hour) were independently associated with SCD risk in the first 6 months after AMI in the thrombolytic era.47 Nevertheless, 10 years later, the presence of more than 10 VPBs per hour was associated only with non-SCD but not with SCD in the contemporary post-infarction population treated with modern primary coronary intervention procedures and widely used β-blockers. Conversely, the presence of NSVT was reported as an independent risk marker for SCD in patients with an LVEF less than 35%.48 It should be stressed that reducing ventricular arrhythmias with pharmacologic agents did not lead to improved survival and, in the case of several drugs, such therapy was associated with worse outcomes.49 Large day-to-day variabilities in NSVT detected by Holter monitoring further limit the use of this parameter as a risk stratifier. Primary prevention of SCD with ICD therapy was introduced by MADIT and MUSTT in patients with documented NSVT and inducibility of ventricular tachyarrhythmias.21,35 Nevertheless, after the MADIT II and SCD-HeFT trials, an EF of 30% or less is considered a sufficient risk stratifier without the need for documenting Holter-detected ventricular arrhythmias or inducible VT.22,24
As previously discussed, LVEF is the most acceptable measure of changes in the myocardial substrate. However, complementary information about the substrate may be obtained from electrocardiology. The parameters of interest include QRS duration and morphology (conduction disturbances), late potentials, and changes in repolarization duration and morphology. A wide QRS interval, particularly in relation to bundle branch block, has been consistently associated with worse prognosis, indicating increased risk of SCD as well as further deterioration of left ventricular function resulting in pump failure and death.31 The majority of recent data based on large clinical trials shows consistent association of prolonged QRS duration with all-cause, short-term, and long term mortality. Nevertheless, the association with SCD is not clear. Recent data based on contemporary population of patients with prior MI, the majority of whom were treated with percutaneous coronary intervention (PCI), showed that prolonged QRS (>120 ms) was associated with higher total and cardiac mortality but not with SCD.50 Discrepancy also exists in terms of QRS duration as a predictor of VT or VF therapy in patients with ischemic cardiomyopathy and ICDs. The Pacing Fast VT Reduces Shock Therapies (PainFREE II) study failed to document any relationship between QRS and VT or VF requiring ICD therapy.51 In patients from MADIT II with ischemic cardiomyopathy, a greater prolongation of baseline QRS was associated only with a trend toward increased benefit from ICD implantation.52 Subsequently, post hoc analysis of the MADIT II cohort showed that a prolonged QRS was associated with SCD risk only in the non-ICD arm, not in ICD recipients.53 It is therefore unclear whether QRS prolongation in the post-infarction cohorts may be considered an independent risk factor for SCD. It is probable that QRS should more likely be considered a surrogate of advanced myocardial disease related to the risk of subsequent events, which are, in turn, related to the development and progression of heart failure, but not as specific for arrhythmic events.
Late potentials refer to low-amplitude signals found at the end of the QRS complex that arise from areas of slow and heterogeneous conduction in a diseased myocardium and may represent and increased risk for subsequent cardiac arrhythmias. In patients with normal QRS duration, the presence of late potentials is a likely risk factor for cardiac events. Various studies in prethrombolytic era documented that abnormal SAECG may predict SCD in patients with prior MI.53,54 Data from MUSTT demonstrated that filtered QRS duration greater than 114 ms was independently associated with the primary study endpoint (arrhythmic death or cardiac arrest) during a 5-year follow-up.55 Patients with an abnormal SAECG had a 28% incidence of primary endpoints compared with 17% in those with normal SAECG (P < .001). The highest risk was found in a patients presenting with a combination of prolonged filtered QRS duration greater than 114 ms and EF less than 30%. Despite several studies that linked abnormal SAECG to arrhythmic events in patients with prior MI, SAECG is now alternatively used to identify low-risk patients, taking into account its high negative predictive value. Furthermore, it seems that in the reperfusion era with high rate of PCI procedures, late potentials lost their predictive value.56 An abnormal SAECG recorded in the early post-infarction period has insufficient predictive power, which seems to be overwhelmed by better predictive value of other ECG parameters (including HRT and TWA). However, data indicate that the combination of abnormalities in the SAECG with positive results of the TWA test might be useful in identifying high-risk individuals in the early post-infarction period.57,58
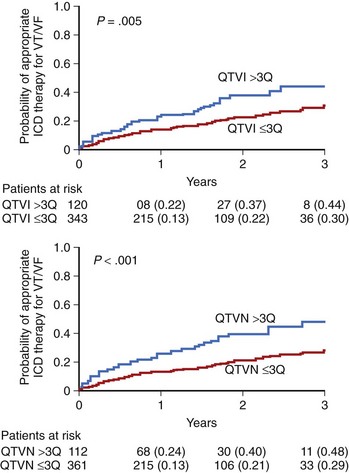
Static measures of QT duration and QT dispersion have been, for years, considered to be risk factors in patients with prior MI; however, their predictive value was usually overwhelmed by clinical covariates. Therefore more and more attention is now paid to dynamic measures of repolarization. The most commonly used method to evaluate QT dynamicity is to assess the relationship between the Q-T interval and the preceding R-R intervals expressed by the QT or R-R slope.29 A steeper slope indicates inappropriate shortening of the Q-T interval at higher heart rate and excessive lengthening of QT during low heart rate—both mechanisms significantly contributing to the risk of arrhythmic events. The dynamicity of the Q-T interval was found to be a potent risk marker of SCD. The Groupe d’Etude du Prognostic de l”Infarctus du Myocarde (GREPI) study demonstrated that a Q-T or R-R interval greater than 0.18 was independently associated with total mortality, with a stronger relationship with sudden cardiac death.59 An increased number of peaks of prolonged QTc interval—for example, the proportion of QTc intervals above the prespecified threshold (QTc >500 ms)—was described as a marker of life-threatening arrhythmias in patients with prior MI.60 A Q-T interval influenced by variety of factors may change in terms of duration as well a morphology. The ECG phenomenon consisting of beat-to-beat changes in repolarization duration and morphology appearing without the 2 : 1 pattern typical for TWA has been termed QT variability. These subtle beat-to-beat changes in T-wave amplitude and shape, as well as in QT duration, may be analyzed by several novel computerized ECG methods. These methods enable detection and quantification of microvolt-level changes, which otherwise remain undetected by the naked eye. Increased QT variability predicted arrhythmic events in the MADIT II population.61 The 2-year risk of VT or VF from Kaplan-Meier curves was twofold higher in patients in the highest quartile compared with those in lower quartiles for QT variability (QTVN) and QT variability index (QTVI) (P < .05 for each) (Figure 72-4). In multivariate Cox analysis, adjusted for significant clinical covariates, top-quartile QTVI and QTVN were independently associated with VT or VF (QTVN: HR, 2.18; 95% confidence interval [CI], 1.34 to 3.55; P = .002; QTVI: HR, 1.80; 95% CI, 1.09 to 2.95; P = .021).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree