Introduction
Rheumatic fever is the most important cause of acquired heart disease in children and young adults worldwide. Initiated by an oropharyngeal infection with group A betahemolytic streptococci (GAS) and following a latent period of approximately 3 weeks, the illness is characterized by an inflammatory process primarily involving the heart, joints, and central nervous system. Pathologically, the inflammatory process causes damage to collagen fibrils and connective tissue ground substance (fibrinoid degeneration) and thus rheumatic fever is classified as a connective tissue or collagen vascular disease. It is the destructive effect on the heart valves that leads to the important effects of the disease, with serious hemodynamic disturbances causing cardiac failure or embolic phenomena resulting in significant morbidity and mortality at a young age.
A number of recent systematic reviews have summarized the effectiveness of primary and secondary prevention of rheumatic fever and the treatment of the acute attack.1–3 The evidence from randomized controlled clinical trials, while dated, is in favor of the effectiveness of antibiotics in the primary (or the treatment of pharyngitis caused by group A streptococci) and secondary prevention of rheumatic fever.1,2 In the treatment of the acute attack, most publications have been observational studies with only a small minority of poorly designed randomized trials.3
In the developed countries of the world, the incidence of rheumatic fever has fallen markedly during the last century. For example, in the USA the incidence per 100 000 was 100 at the start of the last century, 45–65 between 1935 and 1960, and is currently estimated to be approximately 10 cases per 100000.4 This decrease in rheumatic fever incidence preceded the introduction of antibiotics and is a reflection of improved socio-economic standards, less overcrowded housing and improved access to medical care. The current prevalence of rheumatic heart disease in the USA and Japan, 0.6–0.7 per 1000 population, contrasts sharply with that in the developing countries of Africa and Asia where rates as high as 30 per 1000 have been reported.5 As GAS pharyngitis and rheumatic fever are causally related, both diseases share similar epidemiologic features.4 The age of first infection is commonly between 6 and 15 years. Also, the risk for developing rheumatic fever is highest in situations where GAS is more common, for example where people live in crowded conditions.6
Clinical, epidemiologic, and immunologic observations tend to strongly support the causative role of untreated GAS pharyngitis in rheumatic fever.6,7 Beyond this, however, the pathogenesis of acute rheumatic fever (ARF) and clinical heart disease remains unclear and several important and unexplained observations render the management of this important disease extremely difficult. These are:
- individual variability of susceptibility to GAS pharyngitis
- individual variability of development of symptomatic GAS pharyngitis
- individual variability of development of acute rheumatic fever after an episode of GAS pharyngitis
- individual variation in the development of carditis and chronic rheumatic heart disease after an attack of acute rheumatic fever
- the development of chronic rheumatic heart disease (RHD) in patients who have no definite history of acute rheumatic fever.
Streptococcal skin infection (impetigo) is considered not to cause rheumatic fever. However, a report of acute rheumatic fever following streptococcal wound infection8 and the high prevalence of pyoderma with relative paucity of streptococcal pharyngitis in some communities with a high incidence of rheumatic fever has raised questions about the link between streptococcal skin infection and rheumatic fever.9 While effective antibiotic treatment virtually abolishes the risk of rheumatic fever, in situations of untreated epidemic GAS pharyngitis up to 3% of patients develop it.1,7 Worryingly, as many as a third of patients who develop rheumatic fever do so after virtually asymptomatic GAS and in more recent outbreaks, 58% denied preceding symptoms.10 This does not augur well for the primary prevention of rheumatic fever where prompt diagnosis of GAS pharyngitis and treatment are essential.
The virulence of the streptococcal infection is dependent on the organisms’ M protein serotype which determines the antigenic epitopes which are shared with human heart tissue, especially sarcolemmal membrane proteins and cardiac myosin.11 It is these variations in virulence, as a result of M protein variation, which are thought to explain the occasional outbreaks of rheumatic fever in areas of previously low incidence.12 Other factors influencing the risk for rheumatic fever are the magnitude of the immune response and the persistence of the organism during the convalescent phase of the illness.7
Evidence suggests that host factors play a role in the risk for rheumatic fever. In patients who have suffered an attack of rheumatic fever, the incidence of a repeat attack is approximately 50%. A specific B-cell alloantigen has been found to be present in 99% of patients with rheumatic fever versus 14% of controls.13 Certain HLA antigens appear to be associated with increased risk for rheumatic fever. Approximately 60–70% of patients worldwide are positive for HLA-DR3, DR4, DR7, DRW53 or DQW2.14 Such genetic markers for rheumatic fever risk may be useful to identify those in need of GAS prophylaxis. However, in view of the frequency with which these markers occur, they are unlikely to be of practical benefit in the short term.
While there is no specific clinical, laboratory or other test to confirm conclusively a diagnosis of rheumatic fever, the diagnosis is usually made using the clinical criteria first formulated in 1944 by Duckett Jones15 and recently modified by the World Health Organization (WHO).16 The diagnosis is suggested if, in the presence of preceding GAS infection, two major criteria (carditis, chorea, polyarthritis, erythema marginatum, and subcutaneous nodules) or one major and two minor criteria (fever, arthralgia, elevated erythrocyte sedimentation rate, elevated C-reactive protein or a prolonged PR interval on ECG) are present. Evidence of preceding GAS infection, essential for the diagnosis, may be obtained from throat swab culture (only positive in approximately 11% of patients at the time of diagnosis of acute rheumatic fever3) or by demonstrating a rising titer of antistreptococcal antibodies, either antistreptolysin O (ASO) or antideoxyribonuclease B (anti-DNase B).
There has been concern that strict application of the Jones criteria may result in underdiagnosis of rheumatic fever in endemic areas. particularly in the case of recurrent episodes.17 During recurrence of rheumatic activity in a patient with pre-existing RHD, the carditis may precipitate heart failure but may not be possible to diagnose because of lack of information on previous cardiac findings or because valve replacement surgery has been performed. The new WHO criteria thus recommend that a diagnosis of a recurrence of ARF in a patient with pre-existing RHD is possible on the basis of minor manifestations and evidence of recent streptococcal infection.16 Doctors should recognize that the published criteria are guidelines, and are particularly useful in epidemiologic investigations where diagnostic rigor is essential. It is appropriate for clinical judgment to be applied and to supersede guidelines particularly in parts of the world where ARF remains common.17
The most recent recommendations on the prevention of rheumatic fever have been published by the WHO.16
Prevention of rheumatic fever may be considered to be either prevention of the initial attack (primary prevention) or prevention of recurrent attacks (secondary prevention). True primary prevention of rheumatic fever depends more on socio-economic than medical factors. Upgrading housing and other aspects of poverty eradication will do more to eradicate the disease than antibiotic prophylaxis.
Primary prevention
Prevention of the initial attack of rheumatic fever depends on the prompt recognition of GAS pharyngitis and its effective treatment. While it has been demonstrated that therapy initiated as long as 9 days after the onset of GAS pharyngitis can prevent an attack of rheumatic fever,18 early treatment reduces both the morbidity and the period of infectivity.
The first report of the use of penicillin for the treatment of GAS pharyngitis and prevention of most attacks of rheumatic fever was published in 1950.18 Over the following 40 years, attention focused on accurate diagnosis and treatment of GAS pharyngitis. A single dose of intramuscular benzathine penicillin G became the most common mode of treatment and avoided problems of non-compliance. Subsequently, as a result of the pain and possibility of allergic reaction associated with benzathine penicillin G, oral penicillin became the treatment of choice19 and remains so today20 (Class I, Level A). In situations where compliance with a 10-day course of oral penicillin would be unreliable, a single dose of intramuscular benzathine penicillin G would be preferred (dosage 1.2 million u if >27kg, otherwise 600000u).21
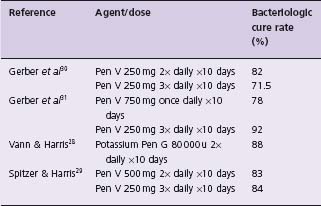
Early studies established a 10-day course of oral penicillin as optimal22,23 and this has been supported in more recent studies.24,25 Shorter treatment periods are associated with significant decreases in bacteriologic cure while longer courses of treatment do not increase cure rate. Current recommendations26 for oral penicillin therapy in children cite a dose of 250 mg two or three times daily. These recommendations are based on trials (Table 53.1) of 250 mg given two, three or four times daily resulting in equivalent cure rates27–30 (Class I, Level A). A dose of 750 mg penicillin once a day yielded significantly worse results than 250 mg three times daily when compared in a randomized study.31 There is no evidence available for optimal doses of penicillin in adults but 500 mg two to three times daily is currently recommended.26
Table 53.1 Cure rates for various penicillin dosage schedules used in treatment of streptococcal pharyngitis
Over the past decade, many trials have been published comparing penicillin VK to a variety of other antimicrobial agents, most commonly cephalosporins and macrolides. This has been prompted by the reported increase in treatment failures with penicillin. It has been suggested that treatment failure rates of up to 38% are possible. This contention, however, has been thoroughly investigated in a study by Markowitz et al32 in which treatment failure rates of penicillin were compared between two time periods, 1953–1979 and 1980–1993. Of the almost 2800 patients with GAS serotyping, treatment failures ranged between 10.5% and 17%, with no significant difference between each time period. It was thus concluded that the overreporting of treatment failures was due to problems with the design of the individual studies.
An increased bacteriologic cure rate for streptococcal pharyngitis by cephalosporins was demonstrated in a meta-analysis of 19 randomized comparisons of a variety of cephalosporins with 10 days of oral penicillin therapy.33 Throat swab cultures were used to determine the presence of GAS and clearance after treatment. The results showed a statistically significant advantage of cephalosporins for which a bacteriologic cure rate of 92% was reported versus 84% for penicillin. The corresponding clinical cure rates were 95% and 89% respectively. It is suggested that the resistance of cephalosporins to penicillinase-producing anaerobes and staphylococci present in the pharyngeal flora may explain these findings. This difference in efficacy would mean that 12–13 patients would require cephalosporin treatment to potentially prevent one penicillin bacteriologic treatment failure (Class II, Level A). More recently, a multicenter comparison of 10-day therapy with cefibuten oral suspension (9 mg/kg/d in one dose) and penicillin V (25 mg/kg/d in three divided doses)34 revealed a bacteriologic cure rate of 91% versus 80% respectively (corresponding clinical cure rates were 97% v 89%). Shorter courses of selected cephalosporins35 (4 or 5 days) have been shown to be effective but current recommendations26 suggest that further study of these regimens is required before their adoption.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


