7
Revision vascular surgery
Introduction
Revision of vascular reconstructions is frequently required beyond the first 6 weeks because of progressive atherosclerosis, graft occlusion, aneurysm formation or infection. Up to 40% of infrainguinal bypass surgery grafts require reintervention within 5 years.1 The same is true for endovascular intervention, where it has been shown that up to 30% of patients need a reintervention within 5 years after endovascular aortic aneurysm reconstruction (EVAR).2–6 Revision surgery requires experience and judgment; open surgery is technically more difficult because of fibrosis and the loss of easily definable tissue planes, which necessitates careful sharp dissection to gain arterial control. The anatomy may be altered by previous operations or interventions, and operating times, blood loss, infection rates and operative risk are therefore increased. Endovascular revision requires careful assessment with regard to when and how to intervene, and especially when to convert to open surgery.
Graft occlusion
Graft thrombosis usually presents acutely but is occasionally foreshown by increasing ischaemic symptoms, ranging from mild claudication to critical ischaemia; asymptomatic graft occlusion can also occur. Occasionally, simultaneous distal embolisation causes digital ischaemia (blue toe syndrome or gangrene). Graft thrombosis with loss of run-off due to distal embolisation is associated with a high risk of limb loss. Whereas graft thrombosis in the first 6 weeks is generally due to technical error or poor run-off, most late occlusions result from intimal hyperplasia within the bypass, progressive inflow or run-off disease (see Chapter 4). Graft stenoses are usually asymptomatic and occur in 20–30% of infrainguinal vein grafts, mostly in the first year.7,8 Stenoses of greater than 70% (velocity > 3 m/s or velocity ratio > 3.0) compromise flow and often occlude if left untreated.9
Factors influencing graft occlusion
These are essentially the quality of the inflow, the run-off and the conduit itself (see Chapter 4).
Arm veins are similar to the long saphenous vein provided that angioscopically detected defects are corrected.18 Preoperative ultrasound mapping is important to identify the best autogenous conduit available.19 A recently published retrospective study of infrainguinal bypasses for critical limb ischaemia (CLI) using arm vein conduits or prosthetic grafts revealed that arm vein conduits, even when spliced, are superior to prosthetic grafts in terms of midterm assisted primary patency, secondary patency, and leg salvage in infrapopliteal bypasses for CLI.20
The risks of ischaemic complications and the need for emergency limb revascularisation are greater with occlusion of prosthetic grafts compared to venous grafts. This is because the thrombus in the prosthetic graft extends into the outflow artery. Vein cuffs at the distal anastomosis reduce the risk of outflow impairment after thrombosis of PTFE grafts (Fig. 7.1).22
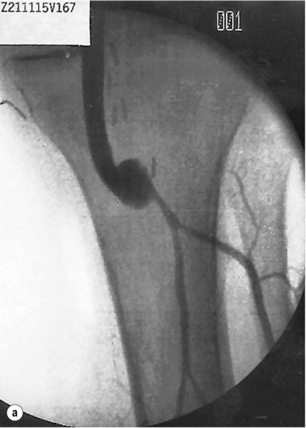
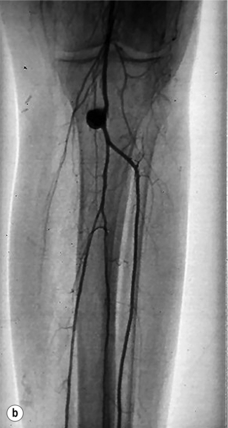
Figure 7.1 Femoropopliteal bypass with venous cuff (Miller cuff) at the distal anastomosis. (a) Perioperative angiography. (b) Preservation of outflow vessels after thrombosis at 3 years.
The quality and number of run-off vessels appear to potentially identify patients at high risk for a poor initial outcome from infrainguinal bypass reconstructions.24,25 Bypasses for gangrene are at higher risk of occlusion than those performed for other indications, probably related to the poorer run-off associated with worsening ischaemia.26
General factors
Raised fibrinogen, hyperlipidaemia, thrombophilias (e.g. protein C, protein S or antithrombin III deficiency, antiphospholipid antibodies) and increased platelet aggregation favour graft thrombosis.30 The role of factor V Leiden mutation is debatable.31,32
Black race and female gender are risk factors for adverse outcomes after vein bypass surgery for limb salvage, with graft failure and limb loss being more common events in black patients, and black women being a particularly high-risk group.33 Hormone replacement therapy potentiates this increased risk.34,35
Management of graft stenosis (the failing graft)
Although there is some discussion about asymptomatic stenosis, it is clear that symptomatic graft stenosis should be treated by either angioplasty or surgical revision. Open surgical revision of infrainguinal vein grafts provides an increased freedom from further reinterventions or major amputation; however, early success rates for endovascular procedures are similar, particularly for non-occluded grafts. With time, endovascular revisions require an increasing number of reinterventions and manifested higher rates of failure.41 Surgical revision is probably more durable in the longer term, but an endovascular approach is actually preferred because of the acceptable short-term patency and a low rate of complications, particularly for late graft stenosis (> 3 months), short (< 2 cm) and single stenoses.42,43
Short graft stenosis, whether midgraft or anastomotic, usually responds well to angioplasty with high inflation pressures (up to 2020 kPa; Fig. 7.2). Cutting balloons have been advocated, but seem to offer only small benefit at the expense of an elevated complication rate.44–46 Stents (preferably self-expandable bare nitinol) should be used only selectively.47
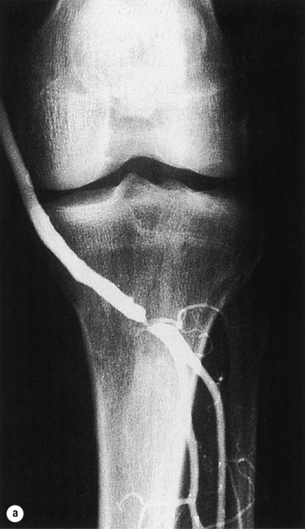
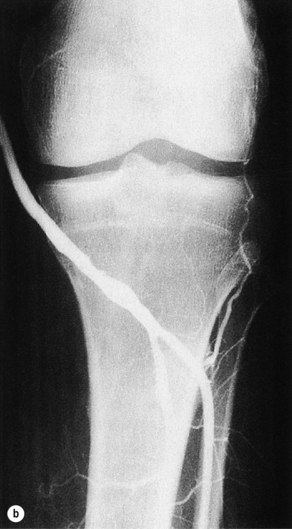
Figure 7.2 Vein graft stenosis near the below-knee popliteal anastomosis (a), successfully treated by balloon angioplasty (b).
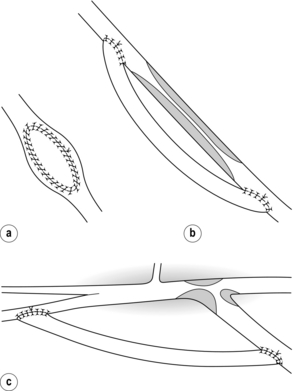
Longer graft stenoses are best treated by open surgery and may be bypassed using the contralateral long saphenous or superficial femoral vein.18 Tibial or distal popliteal anastomotic stenoses, resistant to angioplasty, are best treated by a jump graft to a fresh run-off vessel in order to avoid the scar tissue or adherent tibial veins (Fig. 7.3).
Management of the failed graft
If graft occlusion causes non-disabling claudication, a conservative approach is preferred. Acute subcritical ischaemia allows time for thrombolysis or elective surgery but an anaesthetic paralysed limb demands emergency revascularisation within a few hours (see Chapter 8).
Role of thrombolysis
Although not applicable for suprainguinal grafts, thrombolysis is still frequently used for infrainguinal graft occlusion. The advantages are that redo surgery may be avoided and the graft will be cleared, allowing identification and simultaneous endovascular treatment of the underlying cause of thrombosis. The outflow vessels can also be cleared more effectively than with open surgery. Percutaneous mechanical thrombectomy or the use of high bolus therapy can reduce the duration of the procedure.50,51 If thrombolysis is unsuccessful or reveals a problem that is not amenable to endovascular treatment, then open surgery can be performed with a clear knowledge of the cause of the problem and the state of the run-off. Although thrombolysis has a high initial success rate, it is also characterised by contraindications, haemorrhagic complications, poor long-term patency and persistent ischaemia.52,53 Some therefore advocate reserving thrombolysis to those with associated extensive thrombosis of the outflow vessels.54
Suprainguinal graft thrombosis
A unilateral limb thrombosis of an aortobifemoral graft can often be thrombectomised through the groin even months after the occlusion. Thrombectomy is performed with Fogarty embolectomy catheters, an adherent clot catheter or a ring stripper. During these manoeuvres, the contralateral groin is compressed to avoid embolisation. The underlying cause is most frequently a stenosis at the distal anastomosis, which should be repaired by extension of the graft mostly into the profunda femoris artery (Fig. 7.4). Graft thrombectomy by a femoral approach is usually not possible in the case of bilateral graft occlusion, as the problem is more likely to be related to inflow. Here the graft should be replaced in situ or with an extra-anatomical bypass (axillobifemoral bypass).
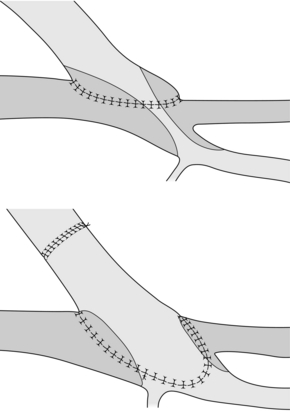
Figure 7.4 Extension graft to one limb of an aortobifemoral graft to treat a stenosis at the profunda femoris origin. In this case the superficial femoral artery is occluded.
The underlying cause of axillofemoral graft occlusion is most frequently an anastomotic or inflow vessel stenosis.55 Thrombectomy can be performed from the groin, but most often an incision along the graft is needed. The underlying cause needs to be addressed. A new graft in unscarred tissues offers the best solution in cases of long-standing thrombosis or when thrombectomy is insufficient. For an occluded femorofemoral graft the same principles apply.
Infrainguinal graft thrombosis
In patients with acute ischaemia, an immediate decision should be taken regarding thrombolysis or open surgery. Prosthetic grafts can usually be thrombectomised by a groin approach if the occlusion is less than 1 week old. Anastomotic stenosis must be corrected, and inflow and outflow vessels need to be checked by perioperative angiography. If necessary, outflow vessels can be selectively approached by an infragenicular approach, and intraoperative thrombolysis may be an adjunct in selected cases (see Chapter 8).56 Venous grafts are usually more difficult to thrombectomise. Here it may be wise to place a new graft, as is the case in prosthetic grafts where thrombectomy is insufficient. If at all possible, a vein conduit should be used. Following thrombectomy for acute ischaemia, four-compartment fasciotomy should be considered to relieve pressure and improve distal perfusion, especially if there is any calf swelling or tenderness preoperatively.57,58
Graft infection
Graft infection is relatively uncommon (1–5%) but has a high amputation and mortality risk.59 In a recent multicentre audit of 55 graft infections, 31% died, 33% underwent amputation and only 45% left the hospital alive without amputation.60 Treatment has therefore to focus on patient survival, eradication of infection, and revascularisation by a method that is durable and does not predispose to further infection.
Causes
Graft infection is thought to occur most commonly by inoculation of bacteria from the patient’s skin at the time of surgery (e.g. skin commensals) or by direct spread in the perioperative period, often secondary to wound breakdown.61 Surgical site infection (SSI) after open surgery for lower extremity revascularisation is a serious complication that is associated with a more than twofold increased risk of early graft loss and re-operation.62
The risk of infection is higher in patients with gangrene, the elderly, obese and those undergoing re-operation during the same hospital admission. Preoperative shaving, open surgical drainage for more than 3 days, operations lasting over 4 hours, emergency surgery, redo surgery, female gender, diabetes, steroids, renal failure, recent angiography and wound haematoma are further risk factors.62,63 Blood-borne bacteria from intravenous lines or systemic infections may also cause graft inoculation and sepsis.
Presentation
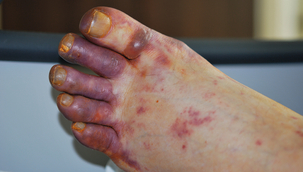
Wound infections after vascular surgery are classified according to the depth of tissue involvement: type 1 involves the skin only, type 2 the subcutaneous tissue and type 3 the graft itself.68 Prosthetic graft infection can present at any time from days to years after surgery with pyrexia, systemic sepsis, local abscesses and sinuses, graft exposure, thrombosis or anastomotic haemorrhage. On rare occasions septic emboli can be the first sign (Fig. 7.5). Septic erosion of exposed vein grafts can occur at any point.
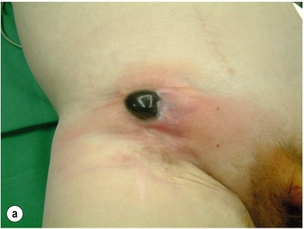
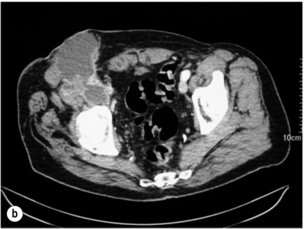
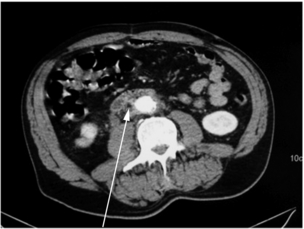
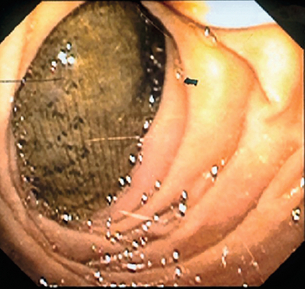
Infrarenal aortic grafts can erode the third or fourth part of the duodenum causing an aortoduodenal fistula, which may present with one or two sentinel gastrointestinal bleeds before the inevitable catastrophic haemorrhage. Occasionally, an aortic graft can also erode any other part of the bowel, including the appendix, and also the ureter. Such aortoenteric erosion will lead to localised peritonitis with retroperitoneal or groin abscesses (Fig. 7.6a, b) and ultimately catastrophic bleeding. The mortality rate of aortoenteric complications is high (> 50%), with recurrent infection or aortic stump blowout in over 25%.69
Bacteriology
Most graft infections are due to skin organisms.70 Staphylococcus epidermidis is the least virulent, producing a biofilm or an infected seroma after months or years. It is difficult to culture, requiring homogenisation of explanted graft material to dislodge adherent bacteria. Staphylococcus aureus is more virulent and usually presents earlier. MRSA infections have a particularly high morbidity and mortality and the incidence unfortunately is increasing. Apart from staphylococci, there are a wide variety of organisms that can cause graft infection. Gram-negative species include Escherichia coli and Pseudomonas aeruginosa, the latter being recognised by a high tendency of anastomotic disruption and bleeding. It may be difficult or even impossible to culture the causative organism, particularly after prolonged periods of antibiotic therapy.
Diagnosis
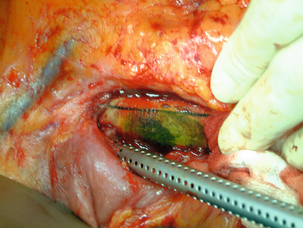
In most cases there is a perigraft collection or sinus. Aspiration of frank pus or turbid fluid from around the graft and the subsequent culture of a causative organism are diagnostic. Computed tomography (CT), magnetic resonance imaging (MRI) or ultrasound usually demonstrate perigraft fluid and inflammation but can underestimate the extent of infection, especially if a sinus is present. A sinogram may be useful in very selective cases, though other modes of imaging will usually suffice. Aortic graft infection is often difficult to diagnose, requiring a high index of suspicion and aggressive investigation. Leucocyte count, erythrocyte sedimentation rate and C-reactive protein are often raised. Persistence of perigraft fluid or perigraft soft-tissue attenuation beyond 3 months or perigraft gas (Fig. 7.7) beyond 4–7 weeks should be presumed to be infection. Perigraft fluid can be aspirated using an image-guided technique and characterised for pathogens, though this does pose a further infection risk.71 One in four anastomotic aneurysms results from graft infection, but this is not always evident from CT. Where doubt exists, indium-labelled leucocyte or positron emission tomography (PET) scanning is occasionally helpful. In aortoenteric fistula, the graft may be seen eroding the duodenum at endoscopy (Fig. 7.8). Definitive confirmation of an infection is sometimes only made at the operation by the presence of pus around the graft and the absence of tissue incorporation (Fig. 7.9).
Management
The mainstay of vascular graft infection management is as follows. First, excision of the graft, as this as a foreign body that may potentiate the infection. Second, wide and complete debridement of devitalised and infected tissue to provide a clean wound in which healing can occur. Third, establishing a vascular flow to the distal bed. Fourth, intensive and prolonged treatment with antibiotics, in order to reduce the risk for sepsis and secondary graft infection.72
Complete graft excision may be mandatory. Partial graft resection commonly requires later replacement.73 Although contrary to conventional concepts, partial or complete graft preservation combined with aggressive drainage and groin wound debridement is an acceptable option for treatment of infection involving an entire aortic graft in selected patients with prohibitive risks for total graft excision.74,75
Simple graft excision without revascularisation can heal infection but usually results in major amputation, even in patients operated on initially for claudication. Revascularisation is traditionally performed by extra-anatomical bypass (axillofemoral graft for infrarenal aortic graft infection, obturator bypass for infection at the groin, lateral bypass for infection of a femoropopliteal graft).59
Arterial allografts are an alternative but they may dispose to late complications such as stenosis, occlusion or aneurysmal degeneration.76,79 There are a few encouraging reports on the use of rifampicin-bonded grafts or silver-impregnated grafts, but they should be reserved for patients with low-grade infections (e.g. Staphylococcus epidermidis).80,81
The duration of postoperative antibiotic therapy is still under discussion. Most authors will accept a period of 2–6 weeks, depending on the causative organism.82
Infrarenal aortic graft infection
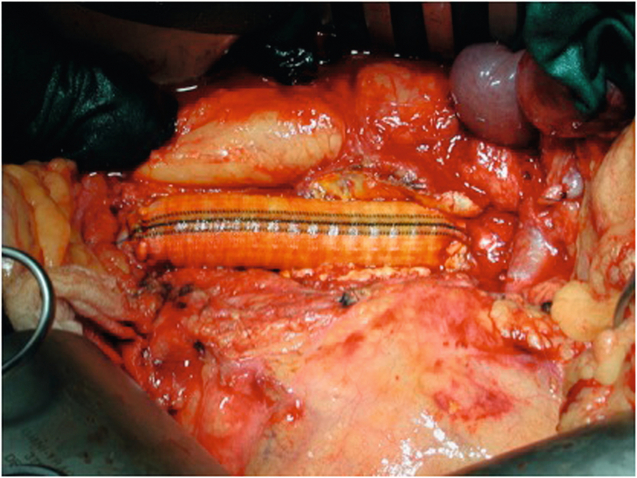
Traditional management of aortic graft infection has comprised extra-anatomical bypass, specifically axillobifemoral bypass, with complete removal of the infected aortic graft. During the last two decades, methods have included debridement of infected tissue, with in situ replacement using cryopreserved allograft, autogenous vein or rifampicin-bonded prostheses (Fig. 7.10).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree