Restrictive Cardiomyopathy
Classification of the cardiomyopathies in general, and restrictive cardiomyopathy in specific, has been controversial. A recent American Heart Association consensus document proposed that cardiomyopathies be classified as either primary (ie, predominantly confined to the heart) or secondary (ie, as part of a generalized systemic disorder).1 A working group of the European Society of Cardiology proposed a classification scheme based on ventricular morphology and function and the familial/genetic backround.2 These proposals offer an alternative to an earlier report from the 1995 World Health Organization/International Society and Federation of Cardiology (WHO/IFSFC) Task Force, which classified cardiomyopathies by dominant pathophysiology or, whenever possible, by etiologic and pathogenetic factors. However, there are several limitations with the earlier approach: (1) the clinical expression of the various cardiomyopathies demonstrates considerable overlap (eg, hypertrophic, infiltrative, and storage diseases are characterized by increased wall thickness and absence of dilation); (2) the anatomic and pathophysiologic expressions of the cardiomyopathies are not static because of cardiac remodeling and the vicissitudes of natural history (eg, amyloidosis and hypertrophic cardiomyopathy can progress from a nondilated to a dilated left ventricular [LV] with systolic dysfunction); (3) the confusion and apparent contradictions that result from a failure to account for recent genetic insights into etiology (eg, restrictive cardiomyopathy may be caused by mutations in sarcomeric genes that have been associated with hypertrophic, dilated, and noncompaction cardiomyopathy) and pathophysiology (eg, some individuals carrying a disease-causing gene mutation fail to express a phenotype or express only a subtle clinical manifestation);3 and, (4) the ambiguities that exist when attempting to categorize heterogeneous disorders by etiology (eg, a myopathic phenotype can have toxic, metabolic, ischemic, or genetic origins).1 Accordingly, it has been suggested that the taxonomy of the cardiomyopathies be reclassified to account for the molecular phenotype using genomic and genetic approaches.4
Restrictive cardiomyopathy refers to either an idiopathic or a systemic myocardial disorder (in the absence of ischemic, hypertensive, valvular, and congenital heart disease) characterized by restrictive filling, normal or reduced LV and right ventricular (RV) volumes, and normal or nearly normal systolic (LV and RV) function. Except for primary nonhypertrophic cardiomyopathy and a few infiltrative diseases (see below), restrictive cardiomyopathies are secondary. These can be noninfiltrative or infiltrative and occur with or without obliteration; infiltration can be interstitial (eg, amyloidosis, sarcoidosis) or cellular (eg, hemochromatosis).
Involvement of the myocardium (or endomyocardium) and ventricular obliteration can occur either in isolation or in the setting of systemic or iatrogenic disease (Table 34–1). Irrespective of the etiology, terminology, or nature of the myocardial process, the ventricles are small (generally <110 mL/m2), and stiff, restricting ventricular filling. Despite normal (or near normal) systolic function at rest, ventricular diastolic, jugular, and pulmonary venous pressures are increased. Elevated atrial pressures produce symptoms of systemic and pulmonary venous congestion (dyspnea, orthopnea, edema, abdominal discomfort), and underfilled ventricles result in reduced cardiac output and fatigue. In patients with restrictive cardiomyopathy as part of a systemic disorder, cardiac symptoms can dominate or overshadow symptoms referable to other organ systems.
| Myocardial |
| 1. Noninfiltrative cardiomyopathies |
| Idiopathic |
| Familial |
| Pseudoxanthoma elasticum |
| Scleroderma |
| 2. Infiltrative cardiomyopathies |
| Amyloidosis |
| Sarcoidosis |
| 3. Storage disease |
| Hemochromatosis |
| Gaucher disease |
| Fabry disease |
| Glycogen storage diseases |
| Endomyocardial |
| 1. Obliterative |
| Endomyocardial fibrosis |
| Hypereosinophilic syndrome |
| 2. Nonobliterative |
| Carcinoid |
| Malignant infiltration |
| Iatrogenic (radiation, drugs) |
Patients with advanced restrictive cardiomyopathy present with signs and symptoms suggestive of heart failure in the absence of cardiomegaly. Physical examination reflects the elevated systemic and pulmonary venous pressure. Striking elevation of the jugular venous pulse and prominent X and especially Y descents are characteristic. A low pulse volume, owing to a reduced stroke volume and tachycardia, can be seen in severe cases. The apical impulse is not displaced, and systolic murmurs of atrioventricular regurgitation and filling sounds marking the abrupt cessation of rapid early diastolic filling can be present; a fourth heart sound (S4) can also be present. Hepatomegaly, ascites, and peripheral edema are also common clinical findings.
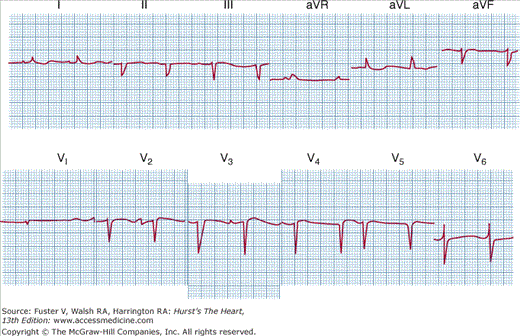
Electrocardiographic abnormalities such as abnormal voltage, atrial and ventricular arrhythmias, and conduction disturbances are frequent, particularly in infiltrative disease; when restrictive cardiomyopathy is caused by amyloid infiltration, low voltage is usual (Fig. 34–1). Atrial fibrillation is common in idiopathic restrictive cardiomyopathy and cardiac amyloidosis.
FIGURE 34–1
Electrocardiogram of a patient with amyloidosis. Note the low voltage, which is in striking contrast to the increased left ventricular wall thickness shown echocardiographically. Reproduced, with permission, from Shabetai R. Restrictive, obliterative, and infiltrative cardiomyopathies. In: Alexander WA, Schlant R, Fuster V, et al, eds. Hurst’s The Heart. 9th ed. New York, NY: McGraw-Hill; 1998:2077.
The chest radiograph usually reveals normal-sized ventricles, though atrial enlargement and pericardial effusion can produce an enlarged cardiac silhouette. Pleural effusions and signs of pulmonary congestion can also be present.
Echocardiographic findings are nonspecific but in many cases are useful to exculpate other, more common causes of heart failure. Although ventricular systolic function at rest is usually preserved, abnormal diastolic function that progresses through the patterns of abnormal relaxation to a restrictive filling can be identified.
Cardiac magnetic resonance imaging (MRI, CMR) can help distinguish between the cardiomyopathies. For example, the cardiomyopathy associated with hemochromatosis produces very low signal intensity because of deposition of iron. In patients with sarcoidosis, late gadolinium enhancement MRI, especially in the basal and lateral segments, identifies cardiac involvement.5 MRI is also excellent for differentiating between restrictive and constrictive disease.6
Endomyocardial biopsy is occasionally useful in patients with severe constrictive/restrictive physiology as it can identify the subset of patients with specific forms of restrictive cardiomyopathy in whom thoracotomy should be avoided.
Differentiation from Constrictive Pericarditis
The differentiation of restrictive cardiomyopathy and constrictive pericarditis remains a challenge. It is important to make the distinction as the treatments differ considerably. Constrictive pericarditis often requires surgical treatment and is usually curable, whereas restrictive cardiomyopathy is treated medically and is incurable without cardiac transplantation. Although several clinical, imaging, and hemodynamic features are helpful in distinguishing restrictive cardiomyopathy from constrictive pericarditis (Table 34–2), considerable overlap and diagnostic confusion exist.
| Restrictive Cardiomyopathy | Constrictive Pericarditis | |
|---|---|---|
| History | Systemic disease that involves the myocardium, multiple myeloma, amyloidosis, cardiac transplant | Acute pericarditis, cardiac surgery, radiation therapy, chest trauma, systemic disease involving the pericardium |
| Chest radiogram | Absence of calcification, massive atrial enlargement | Helpful when calcification persists, moderate atrial enlargement |
| ECG | Bundle-branch blocks, AV block | Abnormal repolarization |
| CT/MRI | Normal pericardium; characteristic MRI patterns | Helpful if thickened (>4 mm) pericardium |
| Hemodynamics | Helpful if unequal diastolic pressures; concordant effect of respiration on diastolic pressures | Diastolic equilibration dip and plateau |
| Biopsy | Fibrosis, hypertrophy, infiltration | Normal |
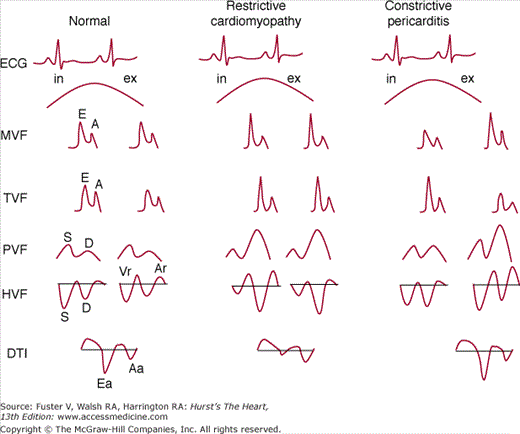
Although motion mode (M-mode) and two-dimensional (2D) echocardiographic findings of constrictive pericarditis (see Chap. 85) can help differentiate these two conditions, Doppler techniques (spectral Doppler, color M-mode, and Doppler tissue imaging) have assumed an important role in characterizing the nature of transvalvular filling and in clinically distinguishing between constrictive pericarditis and restrictive cardiomyopathy. These Doppler flow patterns and the associated respiratory changes are illustrated in Fig. 34–2. In the patient with restrictive cardiomyopathy, mitral valve flow shows an increased E/A wave ratio (≥2) with a short (<150 ms) deceleration time and a short (<70 ms) isovolumic relaxation time (a restrictive pattern of filling) without respiratory variation. The tricuspid valve flow shows an increased E/A wave ratio without respiratory variation, a shortened deceleration time, and a short isovolumic relaxation time that shortens further with inspiration. The systolic/diastolic (S/D) ratio of pulmonary venous flow is <1, atrial reversals are increased (not shown in Fig. 34–2), and there is little respiratory variation. The S/D ratio of hepatic venous flow is <1, and prominent reversals are seen during inspiration. Doppler tissue imaging shows a striking decrease in Ea (<8 cm/s), and the propagation velocity on color M-mode (Vp) is <45 cm/s. In contrast, constrictive pericarditis is characterized by a restrictive filling pattern with respiratory variation of transvalvular and pulmonary venous flows, expiratory hepatic vein flow reversals, and normal or increased Ea and Vp (see Chap. 85).
FIGURE 34–2
Schematic of Doppler flows during inspiration (in) and expiration (ex) in normals, restrictive cardiomyopathy, and constrictive pericarditis. See text for details. A, atrial systolic filling; Aa, late diastolic tissue velocities; Ar, atrial systolic reversals; D, diastolic flow; DTI, Doppler tissue imaging; E, early diastolic filling; Ea, early diastolic tissue velocities; HVF, hepatic venous flow; MVF, mitral valve flow; PVF, pulmonary venous flow; S, systolic flow; TVF, tricuspid valve flow; Vr, V-wave reversals. Reproduced, with permission, from Hoit BD. Restrictive cardiomyopathy. In: Pohost G, O’Rourke R, Shah P, Berman D, eds. Imaging in Cardiovascular Disease. New York, NY: Lippincott Williams & Wilkins; 2000:60-70.
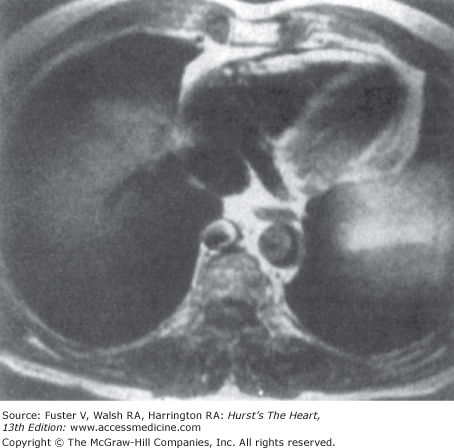
MRI and CT are useful for accurately assessing pericardial thickness (Fig. 34–3); a pericardium >4 mm thick can distinguish the two entities. Ventricular coupling, quantified by the respiratory changes in septal excursion on real-time CINE MRI, can differentiate patients with histologically proven constrictive pericarditis (large septal excursions) from restrictive cardiomyopathy (small septal excursions).6
B-type natriuretic peptide (BNP) levels are markedly elevated in patients with restrictive cardiomyopathy compared to constrictive pericarditis and appear to be a useful noninvasive biomarker to distinguish these conditions.7 Invasive hemodynamics can be helpful (see below), and occasionally a histologic diagnosis is necessary.
It is important to remember that clinical and laboratory testing, including imaging and pathologic studies, can produce results consistent with mixed constrictive pericarditis and restrictive cardiomyopathy; indeed, the two entities can coexist (eg, after mediastinal irradiation or after coronary artery bypass grafting [CABG]). In these cases, a decision to treat conservatively or surgically explore a patient requires experienced clinical judgment.
Cardiac Catheterization
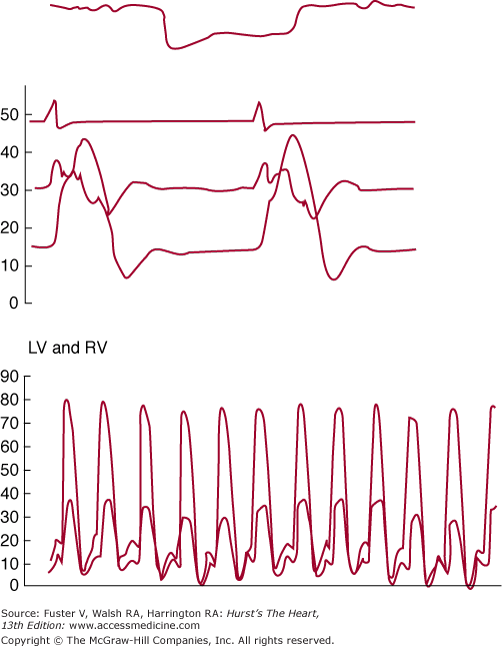
Most patients in whom restrictive cardiomyopathy is a serious consideration should undergo right- and left-sided heart catheterization to document the diagnosis, assess severity, and, in some patients, establish the etiology by means of endomyocardial biopsy. The venous pressure is elevated, and the deep and rapid fall of the right atrial Y descent is striking. During inspiration, the descent of the V wave in the right atrium becomes deeper, steeper, and more pointed, whereas the other waves of the venous pulse and the mean atrial pressure do not vary throughout the respiratory cycle. Patients with constrictive pericarditis generally have a lower RV systolic pressure and an RV end-diastolic pressure greater than one-third of the RV systolic pressure, as opposed to patients with restrictive cardiomyopathy, but these differences are far from absolute. The early portion of diastole is characterized by a deep, sharp dip followed by a plateau, during which no further increase in RV pressure occurs (Fig. 34–4). The hemodynamic features are identical to those of constrictive pericarditis and can cause diagnostic confusion; a higher LV than RV filling pressure strongly favors the diagnosis of restrictive cardiomyopathy rather than constrictive pericarditis. LV systolic pressure is normal, whereas the LV diastolic pressure tracing shows the same abnormalities as those of the RV (see Fig. 34–4). In contrast to constrictive pericarditis, the respiratory changes in right and left ventricular systolic pressures in restrictive cardiomyopathy are concordant (ie, they move in the same direction).
FIGURE 34–4
Top: Right-sided heart hemodynamic data from a patient with amyloidosis recorded with a high-fidelity catheter. From the top tracing down is a respirometer, electrocardiogram, right ventricular (RV) dP/dt, and RV pressure. Note the characteristic dip-and-plateau configuration. Bottom: Simultaneous RV and left ventricular (LV) pressure tracings from another patient with cardiac amyloidosis. In this patient, the typical dip-and-plateau pattern was not present, but during inspiration LV and RV diastolic pressures equilibrated. Reproduced, with permission, from Shabetai R. Restrictive, obliterative, and infiltrative cardiomyopathies. In: Alexander WA, Schlant R, Fuster V, et al, eds. Hurst’s The Heart. 9th ed. New York , NY: McGraw-Hill; 1998:2079.
Left ventriculography usually shows a normal ejection fraction and the absence of major regional wall motion abnormalities. Endomyocardial biopsy is an integral part of the workup of many patients with restrictive cardiomyopathy. When distinction from constrictive pericarditis is particularly difficult, the biopsy can furnish proof of myocardial disease and establish the cause of restrictive cardiomyopathy (eg, amyloidosis), or (by virtue of unremarkable histology) suggest the need for surgical exploration, even in the absence of a thickened pericardium.
Treatment of Restrictive Cardiomyopathy (General Considerations)
Except in certain instances described below (see Specific Restrictive Cardiomyopathic Diseases), the treatment of restrictive cardiomyopathy is empiric and directed toward the treatment of diastolic heart failure. Reduction in the elevated ventricular diastolic pressures produces substantial improvement in pulmonary and systemic congestion, but judicious use of diuretics is warranted in view of the steep pressure-volume relation of the ventricles and the need to maintain a relatively high filling pressure. Vasodilators can also jeopardize ventricular filling and should be used cautiously. Calcium channel blockers are used by some because of their beneficial effect in hypertrophic cardiomyopathies, but improvement in ventricular compliance with their use has not been demonstrated in restrictive cardiomyopathy. Low-dose angiotensin-converting enzyme inhibition can be useful, but orthostatic hypotension, particularly in patients with autonomic nervous system involvement, can be limiting.8 Atrial fibrillation potentially worsens ventricular filling and therefore maintenance of normal sinus rhythm is essential; however, digoxin should be used with caution because of its potential arrhythmogenicity, especially in amyloidosis. β-Blockers are useful in the early stages of disease and control the ventricular response in atrial fibrillation but can exacerbate symptoms later.8 Anticoagulation with warfarin is indicated in patients with atrial fibrillation (and should be considered in patients with dilated atria before the appearance of arrhythmia), valvular regurgitation, and low cardiac output, because of the high incidence of thromboembolic complications. The majority of children with restrictive cardiomyopathy require transplantation within a few years of their diagnosis.9
Specific Restrictive Cardiomyopathic Diseases
A useful classification of the restrictive cardiomyopathies is listed in Table 34–1. This scheme is based on the cardiac compartment predominantly involved (ie, myocardial versus endomyocardial); the myocardial diseases are divided into the noninfiltrative, infiltrative, and the storage diseases, and the endomyocardial diseases are divided into obliterative (ie, endomyocardial fibrosis and the hypereosinophilic syndrome), and the nonobliterative (carcinoid, infiltrative, and iatrogenic).
Although idiopathic restrictive cardiomyopathy is not generally recognized to have a familial predisposition, several small families have been reported.3,10,11 The phenotypes have been variable with both autosomal dominant and recessive patterns of inheritance. Several missense mutations in human cardiac troponin I (one of the genetic causes of hypertrophic cardiomyopathy) have been reported to be responsible for a significant proportion of patients with restrictive cardiomyopathy.7-9 In addition, mutations in other sarcomeric genes (troponin T, β-myosin heavy chain, and α-cardiac actin are reported;2a
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree