Restrictive Cardiomyopathy
James A. Goldstein, MD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Predominant biventricular diastolic dysfunction with lesser impairment of systolic performance.
Predominant biventricular diastolic dysfunction with lesser impairment of systolic performance.
![]() Symptomatic presentation:
Symptomatic presentation:
• Chronic biventricular “backward failure” manifest as dyspnea and peripheral edema (often hepatomegaly and ascites).
• Reduced preload limits cardiac output (fatigue).
![]() Echocardiography: small stiff ventricles, preserved systolic function (until late stages), dilated atria and diastolic dysfunction by Doppler.
Echocardiography: small stiff ventricles, preserved systolic function (until late stages), dilated atria and diastolic dysfunction by Doppler.
![]() Many disorders manifest restrictive “physiology” and must be excluded (eg, hypertrophic cardiomyopathy and constrictive pericarditis).
Many disorders manifest restrictive “physiology” and must be excluded (eg, hypertrophic cardiomyopathy and constrictive pericarditis).
![]() Cardiac magnetic resonance imaging powerful: delineates myocardial infiltration, inflammation, and fibrosis and assesses pericardium, thereby helping establish underlying disorder.
Cardiac magnetic resonance imaging powerful: delineates myocardial infiltration, inflammation, and fibrosis and assesses pericardium, thereby helping establish underlying disorder.
![]() Tissue biopsy may be necessary for definitive diagnosis in some disorders (eg, amyloidosis).
Tissue biopsy may be necessary for definitive diagnosis in some disorders (eg, amyloidosis).
 Overview
Overview
Restrictive cardiomyopathies (RCM) are indolent disabling diseases resulting from pathophysiologic processes that induce predominant diastolic chamber dysfunction with lesser impairment of systolic performance. RCM is characterized by small stiff ventricles with progressive impairment of diastolic filling, leading to the hemodynamic conundrum of low preload but high filling pressures (Figure 24–1). This pattern of diastolic dysfunction leads to dilated atria and elevated mean atrial pressures, resulting clinically in biventricular “backward failure” manifest as pulmonary venous congestion (dyspnea) as well as systemic venous pressure elevation (peripheral edema). Systolic function is preserved in most cases, depending on the underlying cause (at least in the presenting stages of most of the underlying diseases). However, despite intact systolic function, the restrictive constraints on true ventricular preload limit stroke volume, thereby resulting in low cardiac output (fatigue) and ultimately hypoperfusion.
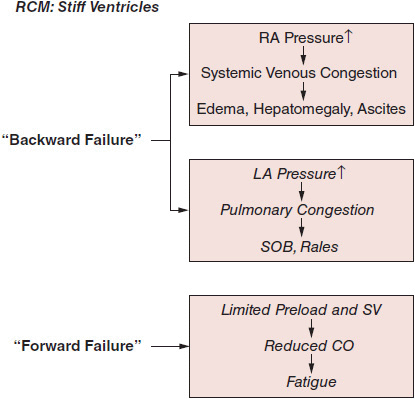
![]() Figure 24–1.Pathophysiology of restrictive cardiomyopathy (RCM). CO, cardiac output; LA, left atrium; RA, right atrium; SOB, shortness of breath; SV, stroke volume.
Figure 24–1.Pathophysiology of restrictive cardiomyopathy (RCM). CO, cardiac output; LA, left atrium; RA, right atrium; SOB, shortness of breath; SV, stroke volume.
The abnormal diastolic properties of the ventricle are typically attributable to abnormalities of the myocardium (infiltration, inflammation, or fibrosis) or the endomyocardial surface (inflammation and scarring). The RCM classification (Figure 24–2) may be most easily considered based on underlying pathophysiologic process as infiltrative (eg, amyloidosis), storage (eg, hemochromatosis), inflammatory (eg, hypereosinophilic syndrome), noninfiltrative (eg, diabetic), or idiopathic. Identification of specific infiltrative processes may have prognostic and therapeutic implications. Of note, secondary restrictive physiology can develop at a late stage in hypertrophic, dilated, valvular, hypertensive, and ischemic heart disease.
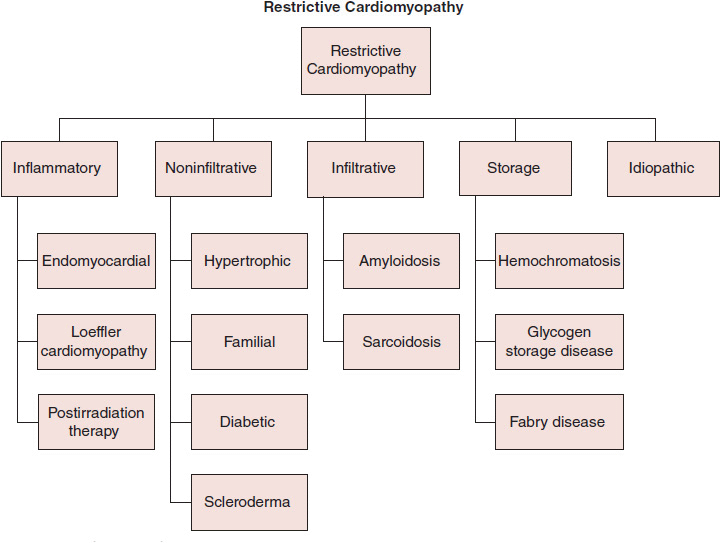
![]() Figure 24–2.Classification of restrictive cardiomyopathies.
Figure 24–2.Classification of restrictive cardiomyopathies.
The vast majority of the underlying disease processes are slowly progressive, but by the time patients develop cardiac symptoms, the disease is advanced pathologically and heart failure progresses rapidly over a short period of time, and the majority die within a few years following diagnosis.
 Pertinent Aspects of Normal Hemodynamics
Pertinent Aspects of Normal Hemodynamics
An appreciation of the physiology of the venous circulations and the dynamic effects of intrathoracic pressure (ITP) and respiratory motion on cardiovascular physiology is critical to understanding the hemodynamic pathophysiology of RCM and differentiating it from other conditions, particularly constrictive pericarditis (CP). Under physiologic conditions, venous return to both atria is biphasic, with a systolic peak determined by atrial relaxation (“X” descent of the atrial and jugular venous pressure [JVP] waveforms) and a diastolic peak determined by tricuspid valve (TV) resistance and right ventricular (RV) compliance (“Y” descent). Respiratory oscillations exert complex effects on cardiac filling and dynamics, with disparate effects on the right and left heart attributable to differences in their anatomic relationships to the respective venous return systems relative to the intrapleural space. Normally, inspiration induces decrements in ITP (–25 to –30 mm Hg), which are transmitted through the pericardium to the cardiac chambers. On the right heart, these decrements in ITP enhance the filling gradient from the extrathoracic systemic veins, thereby augmenting venous return and increasing right heart filling and output. In contrast, the left heart and its tributary pulmonary veins are entirely intrathoracic. Therefore, inspiratory decrements in ITP are evenly distributed, and thus, mitral valve (MV) flow and left ventricular (LV) filling are essentially unchanged throughout the respiratory cycle.
 Pathophysiology
Pathophysiology
The pathognomonic feature of RCM is stiffness of the ventricular walls, which impairs diastolic filling, resulting in limited ventricular preload (and therefore stroke volume) despite increased diastolic filling pressures. Echocardiography (echo) documents small ventricles, often with thick walls. By invasive hemodynamic assessment, RCM is characterized by elevated ventricular diastolic pressures (Figures 24–3 and 24–4), often with a mild diastolic “dip and plateau” or “square root” pattern, a nonspecific indicator of stiff noncompliant chambers, a phenomenon also seen in other conditions such as CP (discussed later). Echo-Doppler demonstrates classic features of impaired diastolic function (impaired myocardial relaxation with increased early LV filling velocity, decreased atrial filling velocity, and decreased isovolumic relaxation time). Ventricular systolic function is typically preserved until the later stages of the underlying disease. Biatrial enlargement reflects ventricular noncompliance and may also result from primary atrial myocardial involvement by the disease process (eg, amyloidosis). Atrial filling pressures are elevated, and the X and Y descents tend to be relatively blunted; in cases in which the atria are primarily involved by the restrictive process, the A wave may be depressed (see Figure 24–3). In RCM, a pattern of “elevated and equalized” chamber diastolic filling pressures indicating pancardiac “stiffness” is common, but this nonspecific pattern is also seen in other diseases, particularly pericardial constraint conditions (eg, CP). However, in RCM, left-sided pressures are typically somewhat higher than right due to the greater intrinsic stiffness of the LV. This difference may be amplified by maneuvers that augment ventricular filling such as volume infusion, leg-raising, or post–premature ventricular contraction increased filling time. Disproportionate left heart stiffness may also result in moderate pulmonary hypertension (a subtle distinguishing feature from CP).
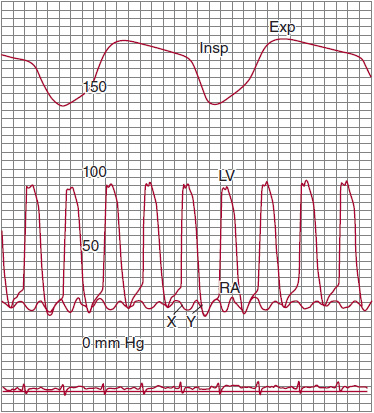
![]() Figure 24–3.Simultaneous recordings of left ventricular (LV) and right atrial (RA) pressures. Note the marked “W” or “M” pattern in the RA pressure tracing with prominent X and Y descents and with no fall with inspiration (Kussmaul sign). The nasal respirometer tracing is also shown. Exp, expiration; Insp, inspiration. (From Higano ST, et al. Cath Cardiovasc Inter.1999;46: 473–86.)
Figure 24–3.Simultaneous recordings of left ventricular (LV) and right atrial (RA) pressures. Note the marked “W” or “M” pattern in the RA pressure tracing with prominent X and Y descents and with no fall with inspiration (Kussmaul sign). The nasal respirometer tracing is also shown. Exp, expiration; Insp, inspiration. (From Higano ST, et al. Cath Cardiovasc Inter.1999;46: 473–86.)
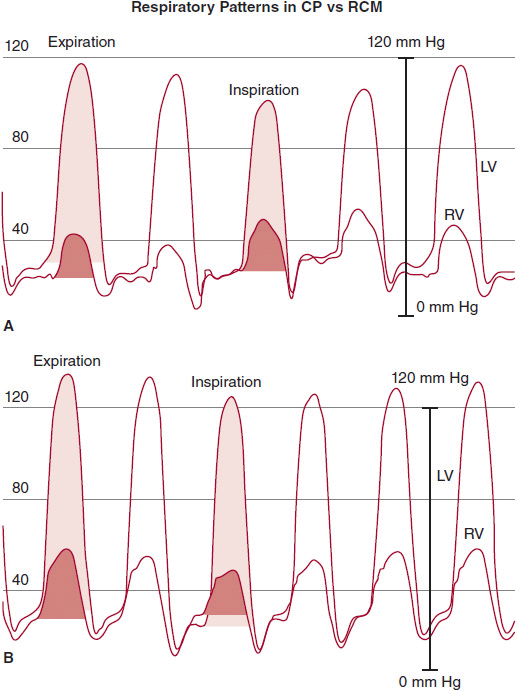
![]() Figure 24–4.Left ventricle (LV) and right ventricle (RV) high-fidelity manometer pressure traces from two patients during expiration and inspiration. Note that both patients have early rapid filling and elevation and end-equalization of the LV and RV pressures at end expiration. A: A patient with surgically documented constrictive pericarditis. During inspiration, there is an increase in the area of the RV pressure curve (orange-shaded area) compared with expiration. The area of the LV pressure curve (yellow-shaded area) decreases during inspiration as compared with expiration. B: A patient with restrictive myocardial disease documented by endomyocardial biopsy. During inspiration, there is a decrease in the area of the RV pressure curve (orange-shaded area) as compared with expiration. The area of the LV pressure curve (yellow-shaded area) is unchanged during inspiration as compared with expiration. (From Talreja DR, et al. J Am Coll Cardiol. 2008;51:315.)
Figure 24–4.Left ventricle (LV) and right ventricle (RV) high-fidelity manometer pressure traces from two patients during expiration and inspiration. Note that both patients have early rapid filling and elevation and end-equalization of the LV and RV pressures at end expiration. A: A patient with surgically documented constrictive pericarditis. During inspiration, there is an increase in the area of the RV pressure curve (orange-shaded area) compared with expiration. The area of the LV pressure curve (yellow-shaded area) decreases during inspiration as compared with expiration. B: A patient with restrictive myocardial disease documented by endomyocardial biopsy. During inspiration, there is a decrease in the area of the RV pressure curve (orange-shaded area) as compared with expiration. The area of the LV pressure curve (yellow-shaded area) is unchanged during inspiration as compared with expiration. (From Talreja DR, et al. J Am Coll Cardiol. 2008;51:315.)
In RCM, inspiratory changes in ITP are fully transmitted through the pericardium to the cardiac chambers. However, chamber noncompliance contributes to a relative lack of respiratory variation in cardiac filling, and therefore, mitral and tricuspid flow velocity during respiration are typically decreased. Because of the lack of pericardial constraint and a relatively noncompliant interventricular septum, there is minimal ventricular interdependence, and thus inspiratory effects on ventricular systolic pressures are concordant (ie, there is little change in peak ventricular systolic pressures with respiration and they move in the same direction) (see Figure 24–4); as will be discussed, the pressure pattern in CP is “discordant.” As RCM progresses and right-sided chambers become less distensible, the respiratory swings in pressure diminish further. At its most severe, noncompliance to inflow results in the “Kussmaul sign,” an inspiratory increase in venous return that cannot be accommodated by the stiff right heart, resulting in inspiratory elevation of JVP (see Figure 24–3). Doppler flow patterns show blunting of the expected physiologic respiratory increases in flow across the TV and minimal change across the MV.
Higano ST, et al. Hemodynamic rounds series II: hemodynamics of constrictive physiology: influence of respiratory dynamics on ventricular pressures. Catheter Cardiovasc Interv. 1999;46: 473–86. [PMID: 10216021]
Kushwaha S, et al. Restrictive cardiomyopathy. N Engl J Med. 1997;336:267–76. [PMID: 8995091]
Mogensen J, et al. Restrictive cardiomyopathy. Curr Opin Cardiol. 2009;24:214–20. [PMID: 19593902]
Oh JK, et al. Diagnostic role of Doppler echocardiography in constrictive pericarditis. J Am Coll Cardiol. 1994;23:154–62. [PMID: 8277074]
 Clinical Findings
Clinical Findings
Patients with RCM suffer symptoms attributable to biventricular diastolic dysfunction. Systemic venous congestion related to right heart noncompliance typically predominates, characterized by pedal edema, abdominal swelling, and gastrointestinal symptoms related to hepatic/bowel congestion (loss of appetite). Physical exam reveals elevated jugular venous pressure often with Kussmaul sign, peripheral edema, and ascites; with advancing disease, hepatomegaly, ascites, and anasarca develop. Patients typically are limited by dyspnea on exertion attributable to left heart diastolic dysfunction. At the bedside, patients will exhibit signs of right heart failure (eg, elevated JVP with edema, ascites), but the precordial exam will be quiet with no evidence of an RV heave, thus pointing away from other causes of right heart failure such as pulmonary hypertension, dilated cardiomyopathy, or TV regurgitation (all of which will manifest an RV heave/lift in the left parasternal area).
In the early stages of RCM, systolic function is typically preserved, although deterioration in contractility may be observed as the disease progresses; the severity and patterns of dysfunction depend on the specific etiology and severity of the underlying disease entity. Despite preserved systolic ventricular function, impaired diastolic filling limits ventricular preload, thereby rendering the stiff heart limited in its ability to increase cardiac output with exercise, resulting in fatigability. Low output combined with autonomic insufficiency characteristic of some RCMs (eg, amyloidosis) renders patients susceptible to symptoms of orthostatic hypotension. Atrial dilation often leads to atrial fibrillation, which further exacerbates diastolic dysfunction due to high heart rate and loss of atrial contraction.
 Etiologies
Etiologies
A. Myocardial Disorders
1. Idiopathic or primary RCM—Idiopathic or primary RCM, often due to hereditary contractile protein mutations, represents approximately 50% of cases and occurs more commonly in older women than men. Wall thickness
is commonly increased by echocardiography, and biopsy typically reveals myocyte hypertrophy often with patchy endocardial fibrosis involving both ventricles. Idiopathic diagnosis can only be established in the absence of other identifiable causes (ie, storage, infiltrative, and inflammatory diseases), many of which induce thick ventricular walls and infiltrative/storage damage on biopsy.
2. Infiltrative disorders
A. CARDIAC AMYLOID—Amyloidosis is the prototypical RCM, arising from a group of disorders resulting in multisystem infiltrative disease with organ involvement, including heart, dependent on the underlying amyloid precursor protein entity. Cardiac amyloid is typically silent until tissue infiltration is extensive, leading to RCM with symptomatically progressive biventricular diastolic dysfunction. Imaging modalities, biochemical tests, and tissue samples establish definitive diagnosis. Echo documentation of thick walls, small cavities, and granular speckled “hyperrefractile” myocardial pattern is nearly diagnostic. Cardiac magnetic resonance imaging (MRI) shows diffuse late gadolinium enhancement throughout both ventricles, particularly the subendocardium. To exclude amyloid of the AL type, monoclonal gammopathy may be evident on urine and serum protein electrophoresis, although immunofixation and assays for serum free light chains are more sensitive. Amyloid deposits can be documented by biopsy of myocardium (by histology, Congo Red staining delineates amyloid deposits between cardiac myocytes); tissue specimens can also be obtained from fat pad or rectum. The general treatment approach is as with any RCM. The mainstay is diuretics to decongest within the limits tolerated by hypotension. These patients are at high risk of thromboembolism, especially with atrial fibrillation, but even in its absence, anticoagulation should be strongly considered. Untreated patients with AL amyloid have a median survival < 6 months after onset of heart failure. Aggressive chemotherapy and stem cell transplantation, followed by cardiac transplantation, have shown promising potential in a small number of cases, but transplantation mortality rate is high and randomized data are lacking.
B. HEMOCHROMATOSIS—Cardiac iron overload, due to heritable disorder or chronic and excessive iron administration, is always associated with involvement of multiple organs, giving rise to the classic clinical presentation of heart failure, cirrhosis, impotence, and diabetes. At pathology, hearts are dilated and ventricular walls thickened. Echo shows a nonspecific mixed pattern of systolic and diastolic dysfunction. The condition is commonly complicated by and may be announced by arrhythmias. Laboratory evaluation is usually diagnostic (marked elevations of plasma iron, serum ferritin, and transferrin saturation, but lower normal total iron binding capacity). Cardiac MRI is a sensitive marker of iron deposition and predictive of future events. Endomyocardial biopsy is definitive, but usually not necessary when biochemical testing is diagnostic. Chelation therapy is appropriate and may limit further damage but is unlikely to reverse existing organ dysfunction.
C. SARCOIDOSIS—This systemic inflammatory condition results in multiorgan noncaseating granulomatous infiltration, typically in the lungs, reticuloendothelial system, and skin. The heart is involved in 20–30% of cases at autopsy, with patchy granulomatous infiltration in discrete areas of the ventricular walls with a predilection for the posterior LV free wall, basal septum, and conduction system. Cardiac infiltrate may result in fibrotic scars and “microaneurysm” formation. Cardiac sarcoid most commonly presents clinically with conduction block or malignant arrhythmias and less commonly with heart failure due to RCM. The electrocardiogram (ECG) may reveal atypical infarction patterns and various degrees of AV block. Echo varies according to disease activity, with wall thickening due to granulomatous expansion but later wall thinning due to fibrosis. Segmental hypokinesia most commonly localizes to mid and basal segments of the LV free wall and upper septum. Owing to patchy involvement, endomyocardial biopsy provides diagnostic evidence in only 25–50% of autopsy-confirmed cases. Cardiac MRI and positron emission tomography (PET) are more sensitive and correlate with disease severity. If treated early, when inflammation predominates and fibrosis is less advanced, anti-inflammatory therapy (steroids and cyclophosphamide [Cytoxan]) may improve cardiac function. In those with arrhythmias, pacemaker and defibrillator therapy should be considered.
D. STORAGE DISORDERS DUE TO HERITABLE DISEASES—RCMs may result from heritable metabolic disorders resulting in myocardial accumulation or infiltration of abnormal metabolic products, producing classic RCM. The recent availability of enzyme replacement in some disorders makes early diagnosis increasingly essential. The most common are glycogen storage disorders (Gaucher and Fabry disease) resulting from lysosomal accumulation in the heart and other organs. Gaucher disease commonly presents with RCM in childhood and is responsive to enzyme replacement therapy or, in extreme cases, hepatic transplantation. In Fabry disease, cardiac involvement is typically manifest in the third or fourth decade of life; the thick ventricular walls mimic hypertrophic cardiomyopathy; differentiation by MRI may be helpful. Other less common heritable storage disorders that may result in RCM include the mucopolysaccharidoses, myocardial oxalosis (related to underling primary hyperoxaluria), and Friedreich ataxia (an autosomal recessive neurodegenerative disorder associated with RCM and/or dilated cardiomyopathy in 90–100% of cases).
B. Endomyocardial Disease
There are two variants of endomyocardial disease, with different pathogenesis but shared and overlapping features. Endomyocardial fibrosis (EMF) refers to a specific syndrome with characteristic geographic epidemiologic features. Other cardiomyopathy syndromes with similar pathologies include Loeffler endocarditis (also called hypereosinophilic endomyocarditis) and other diseases that induce fibrotic changes of the endocardium (eg, carcinoid heart disease).
1. Endomyocardial fibrosis—EMF occurs primarily in tropical regions and in subtropical zones, presents at a young age, and is the most common form of RCM worldwide. EMF is characterized by fibrosis of the apical endocardium of the ventricles. The clinical manifestations are classic RCM.
2. Loeffler “hypereosinophilic” endomyocarditis—This group of disorders results from sustained overproduction of eosinophils, leading to eosinophilic infiltration and mediator release, which damages multiple organs. Diagnosis is established by eosinophil counts > 1500 for at least 6 months. The disease may be primary, but it is essential to search for secondary and potentially treatable causes including leukemia, reactive disorders such as parasite infection, allergies, granulomatous syndromes, hypersensitivity, and neoplastic disorders. Eosinophil-mediated heart damage, detected by echo or MRI, evolves through three stages: (1) an acute necrotic stage; (2) an intermediate phase characterized by thrombus formation along the damaged endocardium; and (3) a fibrotic stage. Treatment is aimed at the underlying disease.
3. Carcinoid heart disease—Carcinoid tumors meta-static to the liver elaborating circulating serotonin (and 5-hydroxyindolacetic acid, its primary metabolite) induce fibrous endocardial plaques in 50% of patients. By echo, plaques are typically seen on the endocardium downstream of the TV, commonly resulting in stenotic and regurgitant valvular lesions (especially TV). The observation that the right heart is preferentially affected reflects inactivation of these toxic substances in the lung. Management of the underlying carcinoid is the focus of treatment. Identical pathology results from exposure to the anorectic drug fenfluramine and its related medications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


