Rest Pain and Nonhealing Ulcers
SUKGU M. HAN and MICHAEL S. CONTE
Presentation
An 81-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents with a slowly enlarging ulcer on the left heel over a 5-month period. The ulcer was preceded by pain that localizes to the base of the toes, described as “burning pain.” The pain is present at rest, worsens when lying in bed or with foot elevation, and is alleviated by dangling the foot in dependent position. In addition, he has a prior history of claudication symptoms, described as a dull achy pain in the left calf with walking two blocks, and relieved with rest. He is a former smoker with a 20 pack-year history, and denies history of stroke or known coronary artery disease. He has never had a deep venous thrombosis. Four months prior to presentation, the patient underwent stenting of the left superficial femoral artery (SFA) and balloon angioplasty of the peroneal artery. Following the procedure, the patient had a temporary relief of leg pain, but the ulcer failed to heal.
On examination, femoral pulses are easily palpable bilaterally. There are no other palpable pulses below this level in either leg. Doppler signals are insonated at both the dorsalis pedis (DP) and posterior tibial (PT) arteries bilaterally. There is an ulcer at the left medial aspect of the heel, measuring 3.5 cm in diameter, with necrotic tissue at the base (Fig. 1). There is no associated erythema or induration surrounding the ulcer. The left foot is cooler than the right, and demonstrates rubor when dependent and pallor on elevation. There is a marked absence of hair on the leg, and the skin appears atrophic, dry, and scaly. He has no carotid or abdominal bruits and no palpable abdominal masses.
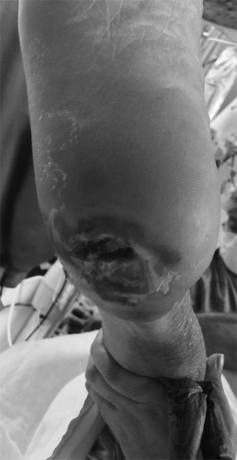
FIGURE 1 Ischemic nonhealing heel ulcer with necrotic base.
Differential Diagnosis
The differential diagnosis of ischemic rest pain includes the pain and paresthesias of diabetic peripheral neuropathy, gout, rheumatologic disorders, osteoarthritis, and common foot conditions such as plantar fasciitis, bone spurs, and benign muscle cramps. The influence of dependency and elevation on this patient’s foot pain and color are nearly pathognomonic of severe chronic limb ischemia and would not be typical of these other conditions. Ischemic rest pain is usually localized to the forefoot and is typically severe enough to require narcotics for symptomatic control. Benign nocturnal muscle cramps in the calf or thigh are a common condition not associated with circulatory disease. Trophic changes of the skin and loss of dermal appendages over the leg and foot are also characteristic of chronic ischemia. In this setting, it is important to examine both feet for additional ulcers that occur at points of pressure (heel) and friction (e.g., between the toes). A history of infection or poorly healing wound that leads to amputation following minor trauma is also suggestive of severe ischemia or poorly controlled diabetes. The reproducible calf pain provoked by ambulation and relieved with rest is characteristic of claudication secondary to arterial occlusive disease. His prior history of leg claudication further supports peripheral arterial disease (PAD) as the etiology of his foot pain. However, it is important to note that many patients who present with signs or symptoms of critical limb ischemia (rest pain, tissue loss) do not relate an antecedent history of claudication, particularly those in whom disease is primarily at the infrapopliteal level.
Discussion
When evaluating a patient with PAD, one should keep in mind the systemic nature of atherosclerosis and assess by history other vascular beds (cerebral, coronary, mesenteric, renal, and aortoiliac) for ischemic symptoms. Recognized risk factors for PAD include age, tobacco use, diabetes, hyperlipidemia, and hypertension. Patients initially presenting with calf claudication are at a relative low risk for limb loss (1% per year). However, claudication, as a marker for overall cardiovascular disease, is associated with an increased risk for cardiovascular death (5% per year). In contrast to claudication, presence of rest pain or tissue loss signifies a more advanced stage of PAD, critical limb ischemia (CLI). Reported 5-year mortality rates for patients with CLI range from 30% to almost 90% in the literature. There is a wide spectrum of CLI disease severity that relates to prognosis for both life and limb. Factors associated with higher mortality and amputation rates in CLI are diabetes, renal disease, tissue loss, and age.
The diagnosis of CLI is based on both the clinical symptoms (rest pain, tissue loss) and objective testing to document diminished lower limb perfusion. Noninvasive vascular laboratory studies such as ankle-brachial or toe-brachial systolic pressure index (ABI or TBI), segmental Doppler pressure (SDP) measurements, pulse volume recordings (PVRs), and duplex ultrasound (DUS) are helpful in distinguishing ischemic pain from other causes of limb pain. In addition, these studies aid in the assessment of the level (aortoiliac vs. femoropopliteal vs. infrapopliteal) and severity of the occlusive disease.
The ABI is a ratio of ankle systolic pressure to the higher brachial artery pressure and is easily performed in the clinic or at the bedside with the use of a handheld Doppler. ABI values generally correlate with disease severity, and an ABI of less than 0.4 is often associated with CLI. ABI measurements can be significantly affected by the presence of tibial artery calcification, which is common in patients with diabetes and renal disease. Inability to achieve flow occlusion with the ankle cuff, absolute ankle pressure greater than 250 mm Hg, or an ABI > 1.4 all suggest false elevation of the ankle pressure due to noncompressible vessels. The toe-brachial index (TBI) is considered more accurate for diagnosing CLI in these settings. A digital cuff is used on the hallux, and a photoplethysmograph is placed on the pad of the digit. TBI < 0.7 is abnormal, and <0.3 generally correlates with severe ischemia.
SDP measurements assess the drop-off in systolic blood pressure due to occlusive disease and can help to localize the level of obstruction. These are measured by inflating the blood pressure cuffs at several locations along the length of the leg. The systolic pressure at which a continuous wave Doppler signal insonated at the ankle returns is compared to the higher of the brachial artery pressures measured. The difference in the pressures measured is attributable to occlusive disease proximal to the blood pressure cuff. Pressure drops of greater than 20 mm Hg are generally considered physiologically significant. PVR are obtained with blood pressure cuffs inflated to 60 to 70 mm Hg placed along the leg in locations similar to SDP measurements. These partially inflated cuffs transmit changes in leg volume that occur with cardiac systole. The waveforms obtained indirectly correlate to the arterial pressure waves generated by the heart during systole. Damping (decrease) of the waveform is related to the presence and severity of occlusive disease proximal to the measuring cuff. PVRs are particularly useful in settings where cuff measurements of systolic pressure may be artificially elevated by noncompressible arteries with medial calcification (e.g., diabetes, renal failure). In patients with toe ulcers or other minor forefoot tissue loss, a PVR at the transmetatarsal level can be helpful to assess healing potential.
DUS is being used with increasing frequency to evaluate PAD. Through examining the velocity characteristics of arterial blood flow along the involved limb, one can localize a significant stenosis by looking for a peak systolic velocity (PSV) elevation as well as spectral broadening. Change of velocity waveform from multiphasic to monophasic across the lesion also signifies hemodynamic significance. The ratio of the PSV recorded across the lesion to that at the normal artery is used to quantify the degree of stenosis. The PSV ratio of 2.0 correlates to greater than 50% stenosis, while a ratio of 4.0 signifies greater than 75% stenosis.
Case Continued
The patient undergoes noninvasive vascular laboratory testing. ABIs are greater than 1.4 bilaterally due to noncompressible, calcified tibial arteries. However, the left TBI is significantly reduced at 0.1. DUS reveals normal velocity waveforms in the left external iliac and common femoral arteries. The previously placed stent in the SFA appears patent; however, there is an elevated PSV to 249 cm/min at the proximal SFA. The velocity waveforms in the popliteal artery show spectral broadening, and loss of multiphasicity. Distally, the DP artery appears patent, but with dampened monophasic waveforms and significant spectral broadening (Fig. 2). These findings are consistent with diffusely diseased left SFA, popliteal arteries, and total occlusion at the infrageniculate level with reconstitution of the DP artery.
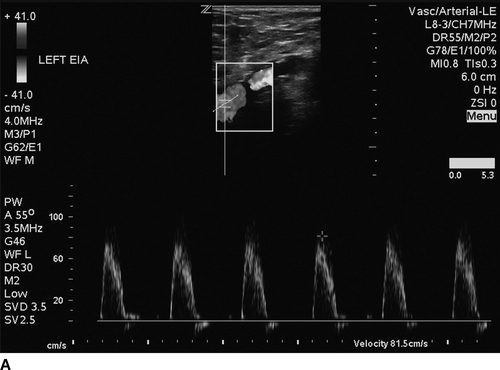
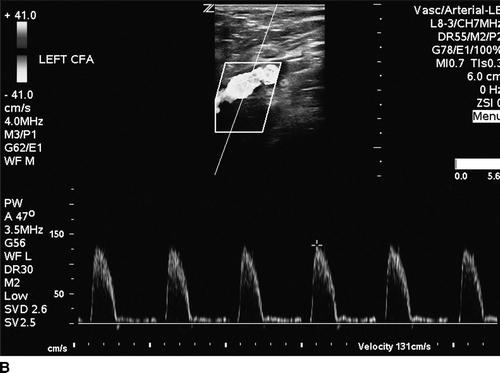
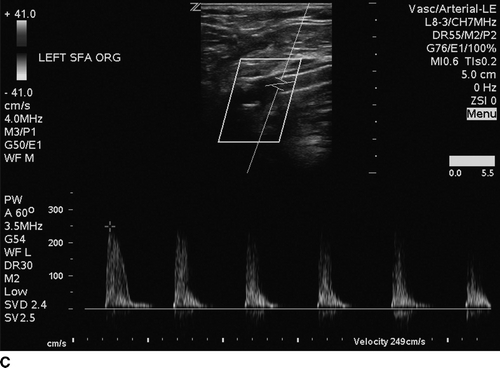
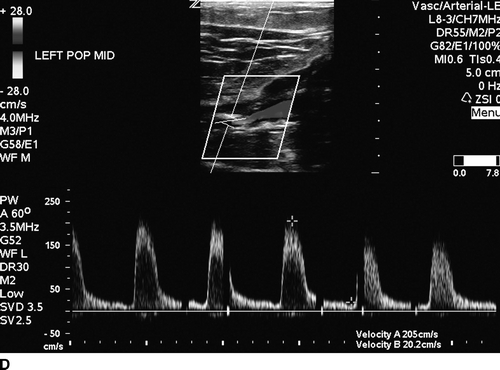
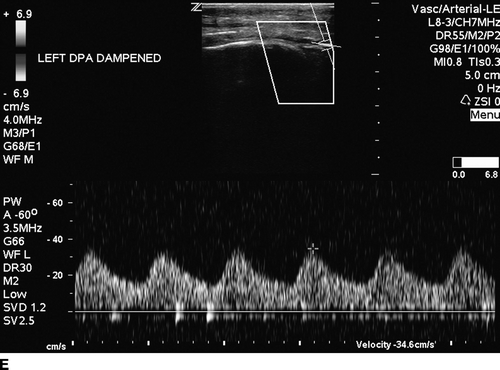
FIGURE 2 Duplex ultrasound of left leg, showing (A) normal, triphasic velocity waveform in the external iliac artery, (B) normal PSV with biphasic waveform in the common femoral artery (C and D) elevated PSV to 249 cm/s and loss of multiphasicity in the proximal to the stented SFA, and the native popliteal artery (E) dampened monophasic signal with spectral broadening in the DP artery.



