Introduction
The primary pathophysiology of acute ST segment elevation myocardial infarction (STEMI) is the total occlusion of a coronary artery, usually through thrombosis of a ruptured or eroded atherosclerotic plaque, with a subsequent lack of blood flow to the myocardium. This then results in a time-dependent “wave-front” of myocardial necrosis beginning at the subendocardium and moving towards the epicardial surface.1 Although in a dog model that has very minimal collateral coronary circulation, the progression to complete transmural necrosis usually takes less than 3 hours, in humans the time may vary anywhere from 2 hours to 12 hours depending on the degree of available collateral coronary circulation, the degree of ischemic preconditioning and whether the thrombotic coronary occlusion is permanent or intermittent. The minimization of the total amount of myocardial necrosis during STEMI has been associated with improved long-term clinical outcomes. Although some evidence exists that myocardial salvage may be accomplished by a variety of pharmaco-logic maneuvers designed to reduce the oxygen demands of the heart, by far the most effective approach has been to produce reperfusion of the occluded artery as fast as possible, by either fibrinolytic therapy or mechanical means. Multiple studies have validated the “need for speed” approach to this effort and justified the mantra that “time is myocardium.”
The benefits of therapy depend on the rate and extent to which myocardial reperfusion is effectively achieved.2–4 Reperfusion is scored by the Thrombolysis In Myocardial Infarction (TIMI) visual2 or frame-counts5 methods supplemented, recently, by a TIMI myocardial perfusion (TMP) score.6 Restoration of TIMI grade 3 (normal) epicardial flow is associated with lower mortality rates than TIMI grades 0-2 (3.7% vs 7.0%). Among those with TIMI 3 flow, lower mortality is associated with TMP grade 3 (0.7%) than with TMP grades 2 (2.9%) or 0-1 (5.4%). The factors differentiating epicardial and myocardial reperfusion are incompletely understood. Platelet and platelet-leukocyte aggregates, distal thrombus embolization and secreted vasoactive and thrombogenic factors have received recent attention, and a variety of targeted therapies, adjunctive to either fibrino-lytic or mechanical reperfusion therapy and designed to further improve myocardial perfusion, are being actively studied.7,8 However, despite the promise shown by multiple agents in the experimental situation, the results of clinical trials have been extremely disappointing and no trial to date has achieved its primary endpoint.9
Although both fibrinolytic and mechanical approaches to coronary reperfusion therapy are presently used, depending on the clinical circumstances, from a historic perspective, fibrinolytic therapy was first developed and validated. In 1933, Tillet and Garner published their discovery of a streptococcal fibrinolysin.10,11 Clinical application of strep-tokinase (SK) to AMI was first reported in 1958.12 From then until 1979, at least 17 studies were published, but AMI pathophysiology was not well understood and results were inconclusive and poorly accepted.13,14 With the establishment of the thrombotic nature of coronary occlusion15 several groups demonstrated the feasibility of clinical fibri-nolysis to achieve early reperfusion under angiographic monitoring (∼75% success with intracoronary (IC) SK) in the period 1976-83.16–19
Randomized studies in AMI followed. Anderson et al reported in 198320 a benefit of early (<4h) IC SK on clinical, enzymatic, and imaging endpoints. Later therapy (at >6 hours) relieved ischemic pain but did not benefit regional myocardial function in another study21 The potential for mortality benefit of IC SK was suggested by subsequent Western Washington and Dutch studies in a few hundred patients.22–24 The logistic difficulties with intracoronary administration stimulated the re-evaluation of IV SK (Schröder et al25). By the mid-1980s, favorable comparisons with IC SK26–28 and a larger outcomes study of IV SK (ISAM)29 established the intravenous route for subsequent clinical trials.
Although intravenous fibrinolytic therapy is certainly superior to placebo in patients who present early with STEMI, there remain a number of problems associated with this approach. First, many patients have significant contraindications to the use of fibrinolytic agents.30 Second, even with the newer fibrinolytic agents, a small but significant risk of intracranial hemorrhage remains and results in death or disability, especially in the very old patients.31 Third, fibrinolytics establish normal TIMI grade 3 flow in only 50–60% of the patients.32,33 Only a third of treated patients have complete resolution of ST segment elevation, and only about 50% have >70% resolution of ST segment elevation 24–36 hours after fibrinolytic administration–a marker of lower mortality.34 Since there are no absolutely reliable clinical signs or symptoms that indicate success of fibrinolytic therapy, it is difficult to evaluate whether the infarct artery is open with the treatment in an individual patient. Even in those patients with successful fibrinolysis, many go on to have reocclusion and reinfarction due to the ongoing vulnerability of the underlying atherosclerotic plaque.35,36
Mechanical reperfusion has the potential to overcome many of these limitations.37 Direct (or primary) PCI, intervention on the infarct artery without prior fibrinolysis, can be done in many patients with contraindications to fibrino-lytic therapy. The overall risk of intracranial bleeding is sig-nificantly lower with direct PCI.38 With the strategy of direct PCI, there is an opportunity to evaluate the overall coronary anatomy, ventricular function, and intracardiac pressures essentially at the time of admission and possibly detect anatomic features or mechanical complications that would require earlier treatment with surgery. Another benefit of direct PCI is the availability of highly trained cardiac cathe-terization laboratory staff if circulatory resuscitation is needed. Additionally, there is a potential to significantly improve the proportion of patients who receive reperfusion with TIMI 3 flow. Early observational studies evaluating the potential of primary PCI were very promising.39 This led to the performance of a number of randomized trials of fibrino-lysis versus mechanical reperfusion therapy in STEMI, most of which demonstrated a significant superiority of primary PCI, especially among patients in which it was logistically feasible to accomplish reperfusion within 90 minutes of presentation (Class I, Level A).40 As will be discussed in more detail later in this chapter, when all things are equal primary PCI is better than fibrinolytics.
Over the past decade, the results of multiple studies have led us to the present state in which, depending on a variety of circumstances, either fibrinolysis or mechanical reperfusion may be the treatment strategy of choice. This chapter will address in detail the evidence behind both approaches and provide guidelines to assist in choosing which form of reperfusion therapy to use when dealing with an individual patient. Potential adjunctive therapies to be used with each approach will be addressed in other chapters.
General mechanisms of action and pharmacologic properties
Fibrinolysis is mediated by plasmin, a non-specific serine protease that degrades clot-associated fibrin and fibrino-gen, disrupting a forming thrombus and facilitating reper-fusion. The fibrinolytic (or “thrombolytic”) agents are all plasminogen activators, directly or indirectly converting the proenzyme plasminogen to plasmin by cleaving the arginine 560-valine 561 bond (Fig. 31.1). Plasmin degrades several proteins, including fibrin, fibrinogen, prothrombin, and factors V and VII. The fibrinolytic agents differ in several properties, as summarized in the text and Table 31.1.
Figure 31.1 Schematic representation of the action of fibrinolytic enzymes. Streptokinase (SK) and urokinase (UK) work predominantly on circulating plasminogen, whereas tissue type plasminogen activator (tPA) and single chain urokinase-type plasminogen activator (scuPA) are relatively clot selective. (Modified with permission from Topol EJ. Clinical use of streptokinase and urokinase to treat acute myocardial infarction. Heart Lung 1987; 16:760.)
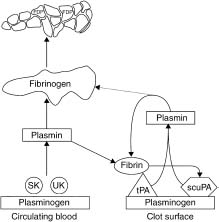
Table 31.1 Comparison of fibrinolytic agents approved by the US FDA for intravenous use
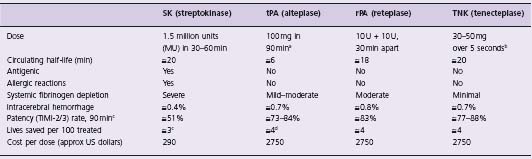
a Accelerated tPA given as follows: 15 mg bolus, then 0.75 mg/kg over 30 min (maximum 50 mg), then 0.50 mg/kg over 60 min (maximum 35 mg).
b TNK is dosed by weight (supplied in 5 mg/mL vials): 60 kg = 6 mL; 61–70 kg = 7 mL; 71–80 kg = 8 mL; 81–90 kg = 9 mL; 90 kg = 10 mL.
c Based on Granger et al49 and Bode et al.89
d Based on the finding from the GUSTO trial42 that tPA saves 1 more additional life per 100 treated than does SK.
Approved fibrinolytic agents
Streptokinase
Streptokinase (SK) is a 415-amino acid bacterial protein sharing homology with serine proteases.41,42 Upon injection, SK forms a 1:l stoichiometric complex with plasmino-gen or plasmin, activating a catalytic site that cleaves plasminogen to plasmin. The half-life of the SK complex is about 23 minutes. SK is antigenic, has little fibrin specificity, and causes substantial systemic lytic effects in clinical doses. Least expensive of fibrinolytics and still widely used globally, SK is administered by short-term (1 h) infusions.
Urokinase
Urokinase (UK) is a native, 2-polypeptide protein derived from human urine or renal cell cultures.43 UK directly converts plasminogen to plasmin. It is non-antigenic and is cleared from the circulation predominantly by the liver with a half-life of 16 minutes. Clinically used doses produce moderately extensive systemic fibrinolysis. Its principal use in North America has been for intra-arterial (including intracoronary) fibrinolysis. It has not been approved for and currently is not available for IV use in acute myocar-dial infarction (AMI).
Tissue-type plasminogen activator (tPA)
Tissue-type plasminogen activator (tPA), a 526-amino acid single polypeptide chain, is the major intrinsic (physiologic) plasminogen activator.44 The marketed form (alteplase) is manufactured by recombinant DNA technology (rtPA). tPA is converted by plasmin to a double-chain form with equivalent fibrinolytic activity.45 tPA has greater activity in the locale of the thrombus and causes less systemic plasminemia, fibrinogenolysis, and proteolysis than SK. tPA is non-antigenic, is inhibited by a circulating plas-minogen activator inhibitor (PAI-I), and is rapidly cleared (half-life about 5 minutes). This short half-life has necessitated bolus/infusion regimens (over 1–3 hours); bolus-only tPA regimens have been tested but abandoned in favor of longer-acting mutant forms of tPA.
Reteplase
Reteplase (rPA) was the first variant (mutant) of tPA to be developed and marketed.46 It is a non-glycosylated, single chain deletion variant consisting only of the kringle 2 and proteinase (plasmin cleavage site) domains of human tPA. Fibrin specificity is lower and half-life longer (14–18 minutes) than tPA, allowing more convenient, double-bolus administration.
Tenecteplase
Tenecteplase (TNK-tPA) is a triple-site substitution variant of tPA: at amino acid 103, threonine (T) is replaced by aspar-agine, adding a glycosylation site; at site 117, asparagine (N) is replaced by glutamine, removing a glycosylation site; at a third site, four amino acids (lysine (K), histidine, arginine and arginine) are replaced by four alanines.47 The first two changes decrease clearance rate (half-life 20 minutes), allowing for single bolus dosing. The third change confers greater fibrin specificity and resistance to PAI-1.
Efficacy of intravenous fibrinolytic therapy
Effects on coronary arterial patency
Because myocardial reperfusion is the postulated mechanism of benefit of fibrinolysis for MI, many angiographic studies have been undertaken to assess patency profiles of the infarct-related coronary artery after fibrinolylic therapy48 Granger et al summarized 14124 angiographic observations from 58 studies.49 Because the extent of myocardial salvage is time dependent, early (0-90 min) patency has generally formed the primary endpoint in these studies. Without fibrinolytic therapy, spontaneous perfusion early after STEMI occurs in only 15% and 21% at 60 and 90 minutes after study entry, respectively, remains unchanged at 1 day, then gradually increases to about 60% by 3 weeks. All fibrinolytic regimens improve early patency rates. At 60 and 90 minutes, streptokinase had the lowest rates (48%, 51%), standard (3-hour) tPA infusions intermediate rates (about 60%, 70%), and accelerated (90-minute) tPA infusions the highest rates (74%, 84%). However, patency rates at > 3 hours were similar for all regimens, and reocclusion rates were higher after tPA than non-fibrin specific (systemically active) agents (13% vs 8%) (I P = 0.002). The GUSTO angiographic study50 embedded within a larger comparative mortality study,42 directly demonstrated that early but not late patency rates accurately predict mortality differences among AMI therapies, providing direct support for the open artery hypothesis of fibrinolytic benefit.
Effect on mortality
By the late 1980s, accumulating clinical trials data provided support for a survival benefit of IV fibrinolysis.51–53 The most important survival trials, comparing fibrinolysis to placebo or standard non-fibrinolytic care, are summarized below.
Gruppo Italiano per lo Studio della Streptochinasi nell’ Infarto Miocardico (GISSI)
This study54 was the first “definitive” mortality trial. Eleven thousand eight hundred and six AMI patients with ST elevation were randomized to receive 1.5 million units (MU) of IV SK over 1 hour or standard therapy. Aspirin was not routinely given. In-hospital mortality was 10.7% in the SK group and 13.0% in the control group, a 17.6% risk reduction (P = 0.0002; relative risk (RR); 0.81). Survival differences remained at 1–2 years.55 Benefit was time dependent and particularly large for treatment within 1 hour of symptom onset (47% mortality reduction, RR 0.49) but was not significant after 6 hours.
Second International Study of Infarct Survival (ISIS-2)
This study56 randomized 17 187 patients with suspected AMI within 24 hours to IV SK (1.5 MU), aspirin (1 62.5 mg), both or neither (placebos) in a 2 x 2 factorial design. The 35-day vascular mortality rate (13.2% for the double placebo group) was reduced 23% by aspirin alone, 25% by SK alone, and 42% by combined aspirin and SK (all P < 0.00001). When both were given early (within 4 hours of symptom onset), a 53% odds reduction was achieved.
Anglo-ScandinavianStudy ofEarlyThrombolysis(ASSET)
This study57 evaluated tPA (alteplase) with heparin versus heparin alone within a randomized, double blind, placebo-controlled design. ASSET enrolled 5013 patients within 5 hours of suspected AMI. Therapies were IV tPA (100mg over 3 hours) plus heparin (5000U N bolus, then 1000 U/h), or placebo plus heparin. The 30-day mortality was lower in the tPA than the placebo group (7.2% vs 9.8%, P = 0.0011). Hemorrhagic risk was acceptable.
Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group
This group58 pooled data from nine controlled trials that randomized 1000 or more patients with suspected AMI. The database consisted of 58 600 patients of whom 6177 (10.7%) died, 564 (1.0%) had strokes, and 436 had major non-cerebral bleeds. The 45 000 patients who presented with ST elevation or bundle branch block (BBB) had an absolute mortality reduction of 30 per 1000 for treatment within the first 6 hours, 20 per 1000 for hours 7–12, and a statistically uncertain reduction of 13 per 1000 beyond 12 hours. These data led to the national guidelines (Class I, Level A) that all STEMI patients should undergo rapid evaluation for reperfusion therapy and have a reperfusion strategy implemented promptly after contact with the medical system.59
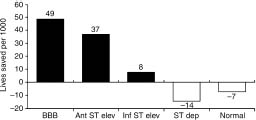
Subgroup analyses by presenting electrocardiogram (ECG) (Fig. 31.2) showed mortality reductions for those with ST elevation (21%, P < 0.00001) and bundle branch block (BBB) (25%) P < 0.01). Benefit was greater for those with anterior (37 lives saved per 1000 treated) compared with inferior (8 per 1000) or other (27 per 1000) AMI sites. The absolute benefit was greater in those with greater risk–for example, BBB (49 lives saved per 1000 treated) and anterior ST elevation (37 per 1000). Those with normal ECGs or with ST depression alone showed no benefit and adverse trends (7 and 14 more deaths per 1000, respectively).
Figure 31.2 The effect of fibrinolytic therapy on mortality (lives saved per 1000 treated) in various patient subsets classified according to admission ECG. Patients presenting with bundle branch block and anterior ST segment elevations derived most benefit from fibrinolytic therapy. Patients with inferior ST segment elevation derived much less benefit, while those with ST depression or normal ECG did not benefit. (Based on data from FTT Collaborative Group.57)
The FTT suggested that proportional mortality reduction was little influenced by systolic blood pressure or heart rate. Benefits also were confirmed for other high-risk groups, including those with prior MI and diabetes.
Benefits of very early (<1 hour) therapy
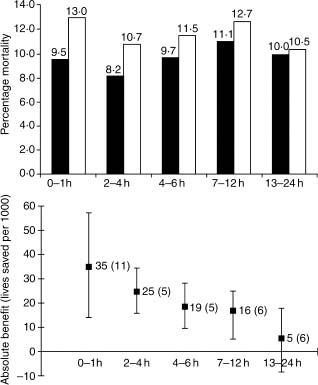
The magnitude of mortality reductions in FTT was dependent on time to therapy from symptom onset. For those with ST elevation or BBB, the absolute benefit was 39 (at 0–1h), 30 (>1–3h), 27(>3–6h), 21 (>6–12h), and 7 (>12–24h) lives saved per 1000 treated (Fig. 31.3).
Figure 31.3 The effect of fibrinolytic therapy on mortality in various patient subsets classified according to duration of symptoms before treatment: (above) mortality in each subgroup of fibrinolytic treated (black bars) versus placebo-treated (white bars) patients; (below) absolute benefit (lives saved per 1000 treated, standard deviation in parentheses) with confidence intervals. (Based on data from FTT Collaborative Group.57)
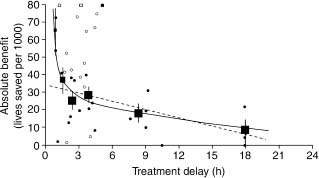
Others also studied the benefits of therapy within 1 hour.60 Boersma et al61 reappraised very early therapy based on a larger database (50 246 patients, derived from all randomized trials of > 100 patients). The absolute mortality reduction for treatment within 1 hour of symptom onset was 65 per 1000. The delay/benefit relation (Fig. 31.4) was non-linear. Other studies have demonstrated that if fibrinolytic therapy is given within 70 minutes of symptom onset, the mortality rate of STEMI can be as low as 1.4%62 and in certain circumstances, very early therapy can even result in aborted myocardial infarction.63 This information has led to a push to provide fibrinolytic therapy in the prehospital setting. The ACC/AHA guidelines have given an indication (Class IIa, Level A) for the establishment of a prehospital fibrinolysis protocol in (1) settings in which physicians are present in the ambulance or (2) well-organized EMS systems with full-time paramedics who have 12-lead ECGs in the field with transmission capability, paramedic initial and ongoing training in ECG interpretation and STEMI treatment, on-line medical command, a medical director with training/experience in STEMI management, and an ongoing continuous quality improvement program.
Figure 31.4 Absolute 35-day mortality reduction versus treatment delay: small closed dots, information from trials included in FTT analysis; open dots, information from additional trials; small squares, data beyond scale of X/Y cross. The linear (34.7– 1.6X) and non-linear (19.4–0.6X + 29.3X–1) closed regression lines are fitted within these data, weighted by the inverse of the variance of the absolute benefit at each data point. The black squares denote the average effects in six time-to-treatment groups (areas of squares inversely proportional to the variance of absolute benefits described). (Reproduced with permission from Antman et al59.)
Benefit of d elayed (>6 hour) therapy
In contrast to earlier therapy, the benefit of fibrinolysis after 6 hours is less certain. The Late Assessment of Thrombo-lytic Efficacy (LATE) study64 enrolled 5711 patients with evidence of AMI between 6 and 24 hours from symptom onset and randomized them to tPA (100 mg over 3 h) or placebo. A 26% relative mortality reduction (8.9% vs 11.9%, P = 0.02) was observed for those treated within 12 hours. The 12–24 hour subgroup showed a non-significant trend to benefit (8.7% vs 9.2% mortality rate). The South American EMERAS collaborative group65 treated 4534 patients with IV SK or placebo within 24 hours after onset of suspected AMI and found a non-significant trend towards a mortality benefit between hours 7 and 12 (SK 1 l.7%, placebo 13.2%). Along with other late treatment trials,58 these have provided the rationale for recommending fibrinolysis for hours 7–12 after the onset of AMI in patients with persistent symptoms and ECG changes.66
Risks of fibrinolytic therapy
Bleeding
Bleeding is the primary risk of fibrinolytic therapy. Intracranial (or intracerebral) hemorrhage (ICH) is the most important bleeding risk, occurring in about 0.5–1.0%, with substantial risk of fatality (44–75%) or disability.67–71 Non-cerebral but not cerebral bleeding risk has benefited by increased fibrin selectivity. The absolute and relative contraindications to fibrinolytic therapy are summarized in Table 31.2.59
Table 31.2 Contraindications and cautions for fibrinolysis in STEMI *
Absolute contraindications Any prior ICH Known structural cerebral vascular lesion (e.g. arteriovenous malformation) Known malignant intracranial neoplasm (primary or metastatic) Ischemic stroke within 3 months EXCEPT acute ischemic stroke within 3 hours Suspected aortic dissection Active bleeding or bleeding diathesis (excluding menses) Significant closed-head or facial trauma within 3 months Relative contraindications History of chronic, severe, poorly controlled hypertension Severe uncontrolled hypertension on presentation (SBP greater than 180 mmHg or DBP greater than 110 mmHg) History of prior ischemic stroke greater than 3 months, dementia, or known intracranial pathology not covered in contraindications Traumatic or prolonged (greater than 10 minutes) CPR or major surgery (less than 3 weeks) Recent (within 2–4 weeks) internal bleeding Non-compressible vascular punctures For streptokinase: prior exposure (more than 5 days ago) or prior allergic reaction Pregnancy Active peptic ulcer Current use of anticoagulants: the higher the INR, the higher the risk of bleeding |
ICH, intracranial hemorrhage; SBP, systolic blood pressure;
DBP, diastolic blood pressure; CPR, cardiopulmonary resuscitation;
INR, international normalized ratio; MI, myocardial infarction.
* Viewed as advisory for clinical decision making and may not be all-inclusive or definitive.
The risk of ICH varies with patient characteristics, the fibrinolytic agent, and adjunctive antithrombotic therapy. Simoons et al70 identified four independent predictors of increased ICH risk: age > 65 years (odds ratio (OR) 2.2; 95% confidence interval (CI) 1.4–3.51), weight <70kg (OR 2.1; 95% CI 1.3–3.2), hypertension on admission (OR 2.0; 95% CI 1.2–3.2), and use of tPA (alteplase) (OR 1.6; 95% CI 1.0–2.5) versus SK. The GUSTO-1 group71 identified seven predictors of ICH: advanced age, lower weight, history of cerebrovascular disease, history of hypertension, higher systolic or diastolic pressure on presentation, and randomization to tPA (vs SK). In contrast, the incidence of non-cerebral bleeding is higher with SK.72
The safety of bolus compared with infusion administration of fibrinolysis for ICH was questioned by a meta-analysis of several different agents.73 However, problems with the meta-analysis have been raised74,75 and large, well-controlled trials of the two bolus agents in general use, RPA76 and TNK-PA,77 have not shown excess ICH rates compared with front-loaded rt-PA.
The critical importance of dose and adjunctive therapies to ICH risk is now realized. Excessive ICH was observed with tPA doses >100mg.48 Excessive adjunctive therapy (for example, heparin, hirudin, glycoprotein IIb/IIIa receptor inhibition) with fibrinolytics also has resulted in unacceptable rates of bleeding including ICH.78-80 In the GUSTO-I trial, the risk of ICH increased with aPTT levels beyond 70 seconds.81 Three concurrent trials77,78,79 were stopped prematurely and reconfigured because of excessive hemorrhage. With lower doses of antithrombins, hemorrhage rates subsequently decreased. Recommendations for adjuvant heparin therapy have been adjusted downward to 60 U/kg bolus (maximum 4000 units) and 12 units/kg/h (maximum 1000 units), adjusted after 3 hours to maintain aPTT at 50–70 seconds for 48 hours.66
Previously, prolonged cardiopulmonary resuscitation (CPR) has been considered a contraindication to fibrinolytic therapy. However, Bottiger et al observed 90 patients with AMI who had out-of-hospital cardiac arrest.82 Patients treated with heparin and tPA more frequently had return of spontaneous circulation (68% vs 44%, P < 0.03), admission to the ICU (P < 0.01), and survival to discharge (15% ~ 8%). Bleeding complications were not problematic.
Allergy, hypotension, and fever
Streptokinase is antigenic and may be allergenic although serious anaphylaxis or bronchoconstriction is rare (<0.2–0.5%).56 In ISIS-369 any allergic-type reaction was reported after SK in 3.6% and tPA in 0.8%; only 0.3% and 0.1%, respectively, required treatment. Angioneurotic and periorbital edema, hypersensitivity vasculitis, serum sickness or renal failure due to interstitial nephritis, and purpuric rashes have been rarely reported, especially after repeat administration.41,56,69 SK may acutely release bradykinin, a vasodilator. The incidence of clinical hypotension after SK (11.8%) was greater than after tPA (7.1%);69 only half of episodes required treatment.
Fever occurs in 5–30% of SK-treated patients. Delayed-type hypersensitivity may provoke fever and may respond to acetaminophen. The role of fibrinolytics in reports of splenic rupture, aortic dissection, and cholesterol embolization is uncertain.
Reinfarction
Although reperfusion may be effected by fibrinolytic therapy, repeat thrombosis and its associated reinfarction is a known, potentially devastating risk after fibrinolytic therapy. In a combined analysis of several fibrinolytic trials including more than 20 000 subjects, Gibson et al83 reported that the frequency of symptomatic recurrent MI during the index hospitalization was 4.2%, and was associated with an increased 30-day mortality (16.4% vs 6.2%, P = 0.001).
Comparative fibrinolytic trials
After establishing the general utility of fibrinolysis in STE-AMI, clinical trials focused on comparisons with new drug regimens. The GISSI-2/International Study Group tria184,85 randomized 20 891 patients with STEM1 <6h to tPA (alteplase, 100 mg/3 h) or SK (1.5 MU/L h) and to subcutaneous (SC) heparin (12 500 U twice daily) beginning 12 hours later, or no heparin. Aspirin and atenolol were given as standard therapies. In-hospital mortality was SK 8.5% and tPA 8.9% (P = NS). ICH rates were 0.5% and 0.8% respectively; other major bleeds were most frequent with SK plus heparin. At 35 days, death or severe left ventricular dysfunction did not differ by fibrinolytic. Delayed, SC heparin added little benefit (RR 0.95; 95% CI 0.86–1.04).
The third ISIS study (ISIS-3)69 randomized 41299 patients with suspected AMI < 24 h old to receive SK (1.5 MU/L h), tPA (duteplase 0.6 MU/kg/4 h) or the streptokinase analog anisoylated plasminogen streptokinase activator complex (APSAC) (30U/3min) and to SC heparin (12500U, 4 hours after beginning thrombolytics and bid) or no heparin. Aspirin (162 mg/day) was given to all patients. The median time to treatment was 4 hours; 88% presented within 6 hours and had ST elevation. Mortality rates at 35 days were: SK 10.6%, APSAC 10.5%, and tPA 10.3% overall, and 10.0%, 9.9%, and 9.6%, respectively, in those with clear indications (P = NS). Similar outcomes also were observed after 6 months. SC heparin tended to improve 1-week mortality (7.4% vs 7.9%, P = 0.06) at the expense of increased bleeding, but mortality rates at 35 days were similar (10.3% vs 10.6%, P = NS).
In comparing fibrinolytic regimens, GISSI-2 and ISIS-3 were limited by the suboptimal use of heparin for short-acting fibrin-selective tPA (SC dosing after a delay of 4–12 hours); treatment was relatively late (mean times >4 hours) and did not require ST elevation (ISIS-3), and tPA was not front-loaded.86–88
These concerns led to the proposal that tPA might be most effective if given in an accelerated dosing regimen and in combination with IV heparin. Table 31.3 summarizes the results of studies comparing various treatment strategies with accelerated dose tPA. The Global Use of Streptokinase and tPA for Occluded Coronary Arteries (GUSTO) study42 randomized 41 021 patients with STEMI <6h to:
Table 31.3 Clinical endpoints in comparative trials of various agents with accelerated t PA
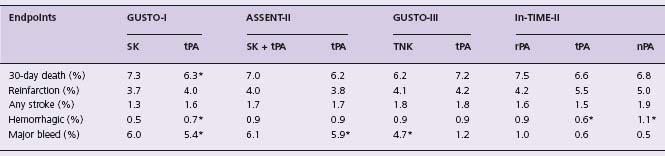
* P < 0.001 for comparisons.
- SK 1.5MU/L h with SC heparin 12500U every 12h starting 4 h after SK
- SK 1.5MU/L h with IV heparin, 5000U bolus then l000 U/h, titrating aPTT to 60–85 seconds
- front-loaded tPA (15mg bolus, 0.75mg/kg (maximum 50 mg) over 30 minutes, then 0.50 mg/kg (maximum 35 mg) over 60 minutes, for a maximum of 100 mg over 90 minutes) and IV heparin as per the SK regimen
- a combination of tPA 1.0mg/kg and SK 1.0MU, administered concurrently over 60 minutes, plus IV heparin. The primary endpoint, 30-day mortality, was lowest with accelerated tPA with IV heparin (6.3%), representing a 14% risk reduction (P = 0.001) compared to the two SK strategies (7.3%), which did not differ. Combined tPA and SK gave an intermediate outcome. The risk of hemorrhagic stroke was higher with tPA (0.7%) than SK (0.5%), but the combined endpoint of death or disabling stroke favored tPA (6.9% vs 7.8%, P = 0.006). Implications of GUSTO for selection of fibrinolytic regimens have been debated.
Comparative trials with bolus fibrinolytics
Reteplase
INJECT (International Joint Efficacy Comparison of Thrombolytics)46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


