Reoperative Cardiac Surgery—General Principles
Damien J. LaPar
Irving L. Kron
CARDIAC REOPERATION
Reoperations for cardiac surgery following prior sternotomy are associated with elevated morbidity and mortality. Major adverse events occurring in the intraoperative and perioperative setting are often predictable but may still occur despite careful attention and surgical detail. As cardiac reoperations are fundamentally different from routine, nonreoperative cases, the general approach to patient selection, preoperative assessment, and surgical technique should be modified from that commonly employed in the routine, nonreoperative setting. In fact, certain advantages may be derived from a protocol-driven approach to reoperations to improve surgical technique and patient outcomes. This chapter reviews general principles that apply to the preoperative and intraoperative management of patients undergoing cardiac reoperations following prior sternotomy.
INCIDENCE AND POSTOPERATIVE OUTCOMES OF REOPERATION
Performance of cardiac operations after prior sternotomy continues to confer an increased risk of operative mortality and morbidity. Recent trends indicate that the performance of cardiac reoperations has been affected by an increase in patient life expectancy and by improved patient outcomes following primary cardiac operations. While evidence demonstrates that the number of patients requiring reoperations for valvular disease has increased, those requiring reoperations for coronary disease has decreased due to improved secondary medical management and evolving surgical techniques. Mortality rates, nonetheless, remain elevated for a variety of cardiac reoperations, including those with prior coronary artery bypass grafting (CABG) and cardiac valve procedures. In a retrospective review of 946 cardiac reoperations performed at our institution from 1995 to 2010, the overall operative mortality was 8.2%. According to recent estimates from the Society of Thoracic Surgeons and other single institutions, mortality for reoperative aortic valve replacements (AVRs) range from 5% to 14%; however, we have recently demonstrated that improved outcomes for reoperative AVRs can be achieved and may, in fact, be comparable to that of primary operations (2.0% vs. 3.5%; P = 0.65). We believe that the improved outcomes for cardiac reoperations observed over time are due to enhanced approaches to patient assessment and selection as well as intraoperative management.
PREOPERATIVE PLANNING
Patient selection and assessment for cardiac surgery following previous sternotomy begins with a comprehensive history and physical exam. A thorough medical history should be conducted to include not only an extensive cardiovascular history but also a detailed history related to prior cardiac surgical interventions or surgical procedures within the thorax and mediastinum. Specifically, special attention should focus upon the type, date, and nature of prior cardiac operations, prior incisions and thoracic approach, and postoperative complications following prior cardiac operations, including respiratory failure, tracheostomy, and sternal wound infections. In addition, a prior history of mediastinal infections, previous chest irradiation, number of cardiotomies, prior pericardiectomy, number, conduit type, and location of previously performed CABGs, and prior implanted valve size and type should be considered. Previous operative notes must be carefully reviewed.
A complete physical exam should be performed. Particular attention should be paid to important findings related to a prior cardiac procedure. In addition to routine physical exam techniques, prior surgical scars on the chest should be assessed for location and extent of healing. Whether a prior cardiac procedure was performed through standard full or partial median sternotomy, thoracotomy, or other minimally invasive approaches often provides valuable insight into the degree of underlying scar tissue that may be encountered upon resternotomy and which may alter existing mediastinal anatomy. Degree of prior wound healing and overall appearance of prior scars may provide additional insight into the setting of prior wound infection, breakdown, or deep sternal wound infection. Potential graft conduit sites should also be considered during the performance of the physical exam for reoperative cases requiring myocardial revascularization. This assessment includes evaluation of both upper and lower extremity venous and arterial conduit sites, including the performance of bilateral Allen tests to assess for collateral flow in patients who may be candidates for radial artery CABGs. Similarly, peripheral vascular access sites for potential femoral or axially cardiopulmonary bypass cannulation should be assessed for patency, prior scars, and ease of access.
Cardiac Catheterization
Standard preoperative evaluation with routine catheterization is fundamental to success for cardiac reoperations. In addition to routine preoperative laboratory analysis, chest roentography, cardiac electrocardiography, and echocardiography, all patients undergoing cardiac reoperations in the setting of prior CABG should undergo coronary angiography within 3 to 6 months of surgery to assess for graft orientation, to determine graft patency, and to establish the contribution of each graft to myocardial perfusion. Coronary angiography also helps to determine the location and proximity of previously placed
bypass grafts in cases of graft adherence to the sternum. This information is crucial to avoid injury and to develop a preoperative plan for myocardial revascularization in the event of intraoperative injury. Timing of preoperative angiography is an issue that affects patient morbidity and mortality. Recent series have demonstrated that contrast loads provided within 24 hours of operation result in significantly higher rates of postoperative renal failure, translating into higher mortality rates and increased hospital lengths of stay and resource utilization. At our institution, we favor cardiac catheterization ≥48 hours prior to operation in order to limit renal dysfunction in the postoperative setting.
bypass grafts in cases of graft adherence to the sternum. This information is crucial to avoid injury and to develop a preoperative plan for myocardial revascularization in the event of intraoperative injury. Timing of preoperative angiography is an issue that affects patient morbidity and mortality. Recent series have demonstrated that contrast loads provided within 24 hours of operation result in significantly higher rates of postoperative renal failure, translating into higher mortality rates and increased hospital lengths of stay and resource utilization. At our institution, we favor cardiac catheterization ≥48 hours prior to operation in order to limit renal dysfunction in the postoperative setting.
Radiographic Evaluation
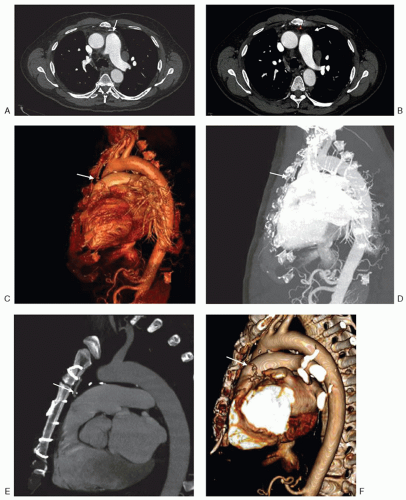
Preoperative computed tomography (CT) should be performed as part of the evaluation of all patients undergoing cardiac reoperations. More specifically, computed tomography angiography (CTA) provides a more complete radiographic assessment of mediastinal anatomy, architecture, and location and status of prior CABG. CTA has many advantages over other imaging modalities due to its ability to provide cross-sectional imaging, which can often be used to provide three-dimensional reconstructions of the heart and mediastinum (Fig. 41.1). Preoperative information that can be obtained from CTA includes the proximity of sternal incision to various mediastinal structures such as the right ventricle, aorta, pulmonary artery, CABG, and other vascular structures crossing the mediastinum. Details related to aortic size and degree of aortic calcification also aid in cannulation and cross-clamping strategies
prior to incision. The use of prior synthetic materials or felt pledgets may also be suggested by the presence of dense adhesions visible on preoperative imaging that may ultimately distort retrosternal anatomy and place cardiac and vascular structures at increased risk for reentry or intraoperative injury. Alternatively, for patients with baseline renal insufficiency of allergies to intravenous contrast, noncontrast CT imaging or magnetic resonance imaging can be performed.
prior to incision. The use of prior synthetic materials or felt pledgets may also be suggested by the presence of dense adhesions visible on preoperative imaging that may ultimately distort retrosternal anatomy and place cardiac and vascular structures at increased risk for reentry or intraoperative injury. Alternatively, for patients with baseline renal insufficiency of allergies to intravenous contrast, noncontrast CT imaging or magnetic resonance imaging can be performed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree