Definition of chronic kidney disease
In 2002, efforts of the National Kidney Foundation (USA) and the international KDIGO group (Kidney Disease Initiative to improve Global Outcomes) led to clinical practice guidelines for the evaluation, classification and risk stratification of CKD.1 CKD was defined as any renal disease lasting for more than three months with histologic or other signs of renal damage. As depicted in Table 69.1, glomerular filtration rate (GFR) may be decreased or normal in affected people.
Table 69.1 Stages of chronic kidney disease (CKD), its approximate prevalence in Western societies and associated cardiovascular risk
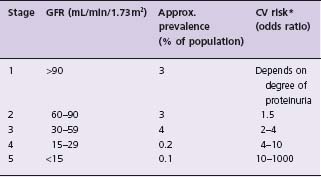
In population studies, GFR was calculated from serum creatinine or cystatin C. The prevalence data are approximated from surveys in North America. The odds of the cardiovascular risk vary substantially with age. For example, the cardiovascular risk of a 20-year-old dialysis patient is about 1000-fold higher than the risk of his age group; at the age of 70 years, it is about 20-fold.
Kidney damage can be detected in several ways including urinary dipstick and sediment, measurement of urine protein, kidney histology, imaging studies or measurement of markers of GFR such as serum creatinine or cystatin C. In the vast majority of cases in adults not presenting with kidney-related symptoms, a reduced GFR or an elevated urinary protein excretion will identify CKD.
Glomerular filtration rate
Normal GFR in young men and women averages about 130 and 120mL/min/1.73m2 respectively.2 After age 40, people tend to lose GFR by approximately 1 mL/min/year; this mean value, however, shows a wide variability, substantially depending on the presence or absence of atherosclerotic vascular disease.3,4 GFR changes little in elderly people without renal and atherosclerotic vascular disease and is regulated in health and disease by various factors. For example, sodium and protein intake increase GFR and sympathetic drive decreases it.
Exact measurement of GFR by inulin or iothalamate clearance is cumbersome and costly and therefore not practical in routine clinical practice. Serum creatinine is the usual clinical indicator of GFR. However, this indicator (an amino acid derivative of 0.11 KD) is imperfect. First, creatinine is excreted by both glomerular filtration and tubular secretion; second, serum creatinine concentration depends substantially on muscle mass such that individuals with very low muscle mass may exhibite terminal renal failure at serum creatinine concentrations barely above 2 mg/dL (175μ mol/L). Third, dietary intake of creatinine with meat may also affect serum levels. Indeed, GFR may decrease by up to 30% without a corresponding increase in serum creatinine above the normal range.
Despite those obvious shortcomings of serum creatinine as a marker of GFR, it is possible to estimate GFR (eGFR) from the serum creatinine using various formulas that include age, sex, race or weight (Box 69.1).2 The Cockcroft–Gault (CG) formula was developed to calculate creatinine clearance from regression computation of data in 249 people with GFR of about 30–130 mL/min, comparing serum creatinine and creatinine clearance. The CG formula tends to overestimate GFR, especially at low GFR. The MDRD (Modification of Diet in Renal Disease study) formula was based on the relationship between serum creatinine and GFR measured by iothalamate clearance in 1628 people with an elevated serum creatinine. This formula appears to be more accurate than the CG formula, at least in ambulatory people with GFR <60mL/min, and has been used in people with several renal diseases and different ethnicity. Of note, the calculation of eGFR improves if assays for serum creatinine are calibrated against a standard; a national program for such calibration is in place in the USA. The MDRD tends to underestimate GFR at levels >60mL/min and may vary from “true” GFR based on inulin clearance by up to 30%. Indeed, above an eGFR of 60 mL/min, the variance of eGFR is so large that many laboratories do not report exact values and just note that the value is > 60. The latter approach appears to be reasonable; otherwise eGFR calculations would inflate the number of people with the label “CKD”. Thus, people with an eGFR >60 mL/min should only be classified as having CKD if there is other evidence of CKD such as increased urinary protein excretion or kidney damage detected with imaging or histologic studies.
Cystatin C in serum is an alternative to creatinine; it is a 13 KD protein, freely filtered by the glomerulus with extensive tubular reabsorption and catabolism. Its generation as a nuclear degradation product appears to be more stable than the generation of creatinine. Recent data suggest that serum concentration of cystatin C may be a better marker of GFR than creatinine-based eGFR at GFR >60mL/min. Cystatin C measurements are much more expensive than creatinine (6–10-fold difference) and further research is necessary to establish the role of cystatin C in clinical medicine.
BOX 69.1 Formulas to estimate glomerular filtration rate
Modification of Diet in Renal Disease (MDRD) formula:
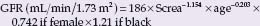
Cockcroft and Gault formula:
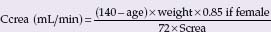
Formulas derived from serum cystatin C:
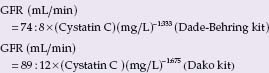
Screa, serum creatinine; age in years, weight in kg; Ccrea, creatinine clearance; GFR, glomerular filtration rate. There are several formulas developed from the MDRD data set. Above we give the most often used abbreviated formula. Slightly more exact is the following MDRD formula: GFR (mL/min/1.73 m2)= 170 × Screa ~0.999 × age ~0176 × Surea ~0.1760 × Salbumin +0318 × 0.762 if female × 1.180 if black.
Urine protein
If the kidney leaks proteins, this may reflect underlying renal disease or, in the case of small amounts of albumin, generalized endothelial dysfunction. Common renal diseases are glomerulonephritis, inherited diseases such as polycystic kidney disease, diabetic nephropathy and nephrosclerosis due to smoking or hypertension. Laboratories typically measure either all proteins in the urine or albumin alone; dipsticks detect only albumin. A variety of assays are available and day-to-day variation of urinary protein excretion is substantial, depending on factors such as posture and physical activity. Therefore, urinary protein excretion should be measured more than once to detect kidney damage. Moreover, when urinary protein excretion is measured, it should either be expressed as a ratio to urine creatinine (to account for urine concentration) or as a timed specimen (e.g. per 12 or 24 hours).1
Urinary protein excretion is a very strong predictor of cardiovascular risk. This association is progressive; that is, the risk of incident cardiovascular outcomes increases with urinary protein excretion, even within the “normal” range.5 The term microalbuminuria stems from studies in diabetes and indicates a threshold at which the risk for overt diabetic nephropathy (macroalbuminuria and/or renal insufficiency) suddenly increases severalfold. Such a threshold does not exist in the relationship between urinary albumin excretion and cardiovascular risk. As the term microalbu-minuria is well established in clinical medicine, we use it here to describe renal damage and its association with cardiovascular risk.
Low GFR and high urinary protein excretion often coincide. In many studies, however, only one of these standard markers of renal damage is reported, usually serum creatinine. The few studies that documented both parameters suggest that both of these renal-related cardiovascular risk factors are additive and independent of each other.6,7
When to measure eGFR and urinary protein excretion?
There is no general consensus regarding when to measure kidney-related cardiovascular risk factors. In general, serum creatinine is measured whenever blood chemistry is ordered. Guidelines support the measurement of serum creatinine whenever a cardiovascular risk profile is evaluated in a person. Urinary protein excretion is usually ordered in the evaluation of kidney disease or at regular intervals in people with diabetes. In a cardiovascular setting, both eGFR and urine protein may be ordered if one is unsure whether to start therapies to prevent cardiovascular disease in a given patient. The presence of increased urinary protein excretion or a low eGFR may then justify such therapy. However, data regarding the additional value of proteinuria and eGFR on top of classic cardiovascular risk are lacking, and some analyses suggest that these measurements are of limited value after accounting for classic cardiovascular disease risk factors.
Population-wide impact of cardiovascular risk associated with renal dysfunction
GFR
A recent systematic review of 39 studies comprising approximately 1.4 million people followed for a median of 4.5 years reported that cardiovascular mortality was increased with reduced eGFR in all but three studies. The univariate risk increase was twofold (range 1–5-fold); this risk was somewhat attenuated after multivariate adjustments.8 A low eGFR is not consistently associated with cardiovascular risk in the general population8 due to the low number of cardiovascular outcomes and concomitant low power. However, one study of >1 million participants of the general population in California reported a continuous increase in cardiovascular risk (adjusted hazard ratio) for eGFRs between 45 and 59 mL/min to <15mL/min from 1.4- to 3.4-fold9 compared to an eGFR of >60mL/min (Fig. 69.1); the risk increase for mortality and hospitalization was steeper. Of note, the prevalence of an eGFR <60mL/min in the general population, especially above age 50, is in the range of 5–10%.10 Conversely, in people with known cardiovascular disease, the prevalence of an eGFR <60mL/ min is higher than in the general population, in the 15–20% range.7,10
Figure 69.1 Relationship between estimated glomerular filtration rate (eGFR) and cardiovascular outcomes in a general population of >1 million people of a health maintenance organization in California, followed for 2.5 years. Abscissa: GFR estimated from serum creatinine at baseline. Ordinate: relative risk % for death (light gray), for a major cardiovascular event (black) and for hospitalization (gray). Risk of people with a baseline GFR >60 mL/min/1.73 m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


