Renal Artery Stenosis: Endovascular Therapy
VENKATARAMU N. KRISHNAMURTHY and PAULA M. NOVELLI
Presentation
A 62-year-old male presents with a 4-week history of hypertension (210/112 mm Hg). He is a current smoker with a 70-pack-year history of tobacco use. His past medical history is significant for coronary artery disease (CAD), carotid endarterectomy, and peripheral arterial disease (PAD) (aortofemoral bypass surgery for aortoiliac occlusive disease). Initially, he received diuretics and beta-blockers for his hypertension. Because of poor control of hypertension, an angiotensin-converting enzyme (ACE) inhibitor was later added. During this period, his renal function deteriorated (serum creatinine increased from 0.8 to 2.1 mg/dL). The ACE inhibitor was then discontinued with improvement in renal function.
Differential Diagnosis
Essential hypertension is the cause of hypertension in more than 95% of cases. In less than 5% of patients, an underlying cause is identified (Table 1). Renovascular hypertension (RVH) is the most common secondary cause. Nonessential hypertension is usually suspected in patients with recent onset of severe (greater than 200 mm Hg systolic and/or greater than 100 mm Hg diastolic) hypertension. Other indicators of RVH are listed in Table 2. In the current case, RVH is supported by the patient’s significant past history of PAD, CAD, and acute deterioration of renal function after administration of an ACE inhibitor.
TABLE 1. Secondary Causes of Hypertension

TABLE 2. Clinical Findings Indicative of Renal Artery Stenosis as a Cause of RVH

Discussion
Etiology and Prevalence of RVH
Atherosclerotic renal artery stenosis (ARAS) is the most common etiology (80% to 90% of cases) and is typically ostial (at the ostium and within the proximal 1 cm of the renal artery) (Fig. 1). Major risk factors for ARAS include advanced age, female gender, hypertension, CAD, PAD, chronic kidney disease, diabetes, tobacco use, and hypercholesterolemia. ARAS affects 6.8% of people older than 65 years. Prevalence is higher in patients with congestive heart failure (54.1%), hypertension and diabetes (20%), PAD (25.3%), abdominal aortic aneurysm (33.1%), and end-stage renal disease (40.8%) (Table 1).
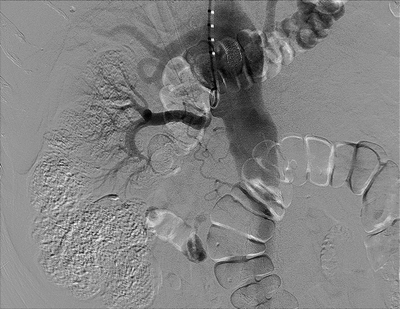
FIGURE 1 Flush aortogram from brachial approach. Pigtail catheter is seen in the proximal aorta. There is severe ostial stenosis of the right renal artery and nonfilling of the left renal artery. Incidental note made of stent in the celiac artery.
TABLE 3. Key Technical Steps and Potential Pitfalls
Fibromuscular dysplasia (FMD) is the second most common cause (10%) of RVH. FMD is a nonatherosclerotic, noninflammatory disease of medium-sized arteries characterized by fibrodysplastic changes that may cause stenosis, aneurysm, dissection, and/or occlusion. FMD is associated with smoking and is transmitted in an autosomal dominant manner with incomplete penetrance. FMD most frequently occurs in women between the ages of 20 and 60 years. FMD is classified based on the arterial wall layer affected: intima, media, or adventitia. The most common histopathologic type is medial fibroplasia, which angiographically has the classic “string of beads” appearance resulting from alternating areas of stenotic fibrous webs and poststenotic dilatation (Fig. 2).
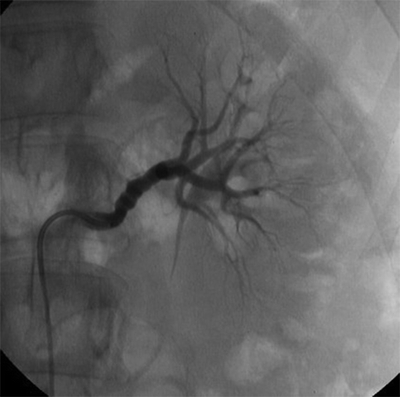
FIGURE 2 Selective renal arteriogram images of the left renal artery in a young woman with severe uncontrolled hypertension demonstrates classical beaded appearance of the renal arteries consistent with FMD.
Other vascular pathologies (vasculitis, neurofibromatosis type 1, aortic wall hematoma, or aortic dissection) account for the remainder 1% to 2% cases of RVH. RAS caused by Takayasu’s arteritis is rare in the western world but common in Asia.
Workup of Patients with RVH
Duplex ultrasonography (DU) is the most widely used test in the diagnosis and follow-up of patients with RAS. Major criteria for diagnosis of significant RAS (greater than 60% narrowing) are a peak systolic velocity (PSV) in the main renal artery above 180 cm/s associated with poststenotic turbulence and/or a renal artery to aorta PSV ratio above 3.5. Segmental waveforms within the arcuate vessels such as dampening (“parvus” and “tardus”) and resistive index (relative flow velocities in diastole and systole within small vessels in the kidneys greater than 0.015) provide indirect evidence, but the accuracy is limited. Overall, major limitations of DU are low specificity and accuracy (60% to 90%) as the test is highly operator dependent and difficulties of obtaining adequate studies because of obesity, overlying bowel gas, multiple renal arteries, segmental renal artery/branch stenosis, and renal ptosis.
Computed tomographic angiography (CTA) provides excellent resolution and can outline the vasculature in a manner similar to digital subtraction angiography (DSA). The volume data can be post-processed by multiplanar formatting, maximum intensity projection (MIP), and 3D volume rendering (Fig. 3A and B). CTA has high sensitivity (98%) and specificity (94%) for proximal renal lesions as seen in ARAS. Due to higher resolution capability, CTA is better than is magnetic resonance imaging angiography (MRA) in distinguishing ARAS from FMD. CTA has the best accuracy among the noninvasive imaging tests in the diagnosis of FMD; reported sensitivity is 64% to 99% and specificity 89% to 98%. Disadvantages are exposure to ionizing radiation, potentially nephrotoxic iodinated contrast medium, and difficulty in assessment of RAS in the presence of heavy calcium burden in the atherosclerotic plaque.
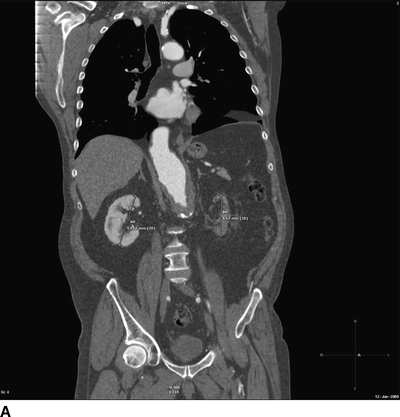
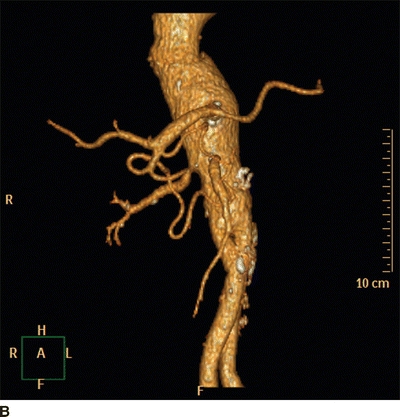
FIGURE 3 A: Coronal reformatting of the CT angiogram image from the patient in Figure 1 shows measurements of the kidney lengths. The right kidney demonstrates good enhancement of the cortex, but the left kidney is nonenhancing. B: Three-dimensional (3D) volume rendering images from the same patient in anteroposterior and posteroanterior views demonstrate severe renal artery stenosis.
Three-dimensional gadolinium-enhanced MRA, like CTA, is best at evaluating the proximal RAS (83% to 100% sensitivity and 92% to 97% specificity). MRA has an extremely low false-negative rate in determining global renal ischemia (bilateral RAS or RAS in the solitary, functional kidney); therefore, the presence of normal main renal arteries can effectively rule out ischemic nephropathy. Limitations of MRA are its tendency to overestimate the severity of stenosis in the presence of calcified atheromatous plaque, motion artifact due to the patient’s inability to hold still due to long scan times or claustrophobia, and presence of metals that disturb the magnetic field such as surgical clips, vascular stents, and prostheses. Another significant disadvantage is the risk of nephrogenic systemic fibrosis (NSF), a debilitating and sometimes fatal skin and muscle condition, associated with the administration of gadolinium-based contrast medium in patients with renal insufficiency. The U.S. Food and Drug Administration has issued a “black box” warning effectively eliminating its use when estimated GFR is below 30 mL/min/1.73 m2.
Physiologic and functional studies such as plasma renin activity assay, captopril plasma renin test, renal vein renin assay, and ACE-inhibitor augmented scintigraphy are aimed at detecting the activation of and the resultant effects of the renin-angiotensin system. These studies have the potential to detect hemodynamically and physiologically significant RAS and to predict the clinical response to revascularization. The major limitation is the low accuracy, particularly in the presence of renal insufficiency or bilateral disease and the requirement for the discontinuation of antihypertensive medications affecting the renin-angiotensin system. Therefore, these studies are most appropriate in patients with suspected FMD or uncomplicated ARAS with normal renal function.
Catheter Angiography (DSA) in combination with intra-arterial pressure measurements is an invasive procedure and is considered the gold standard test for evaluation of RAS. DSA has many advantages such as excellent resolution, very high accuracy, and the ability to diagnose and treat RAS during the same session. A ≥70% RAS with a trans-stenotic peak systolic gradient of ≥20 mm Hg is considered hemodynamically significant. Major disadvantages of DSA are its invasive nature, risk of contrast-induced nephropathy (CIN), cholesterol embolization, and high cost.
In patients with renal insufficiency, use of iodinated contrast can potentially be diminished or eliminated by use of carbon dioxide gas as alternative contrast medium (Fig. 4). Intravascular ultrasound (IVUS) may also be used as an adjunct in such situations.
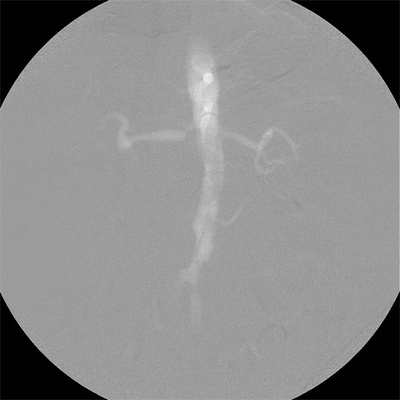
FIGURE 4 Aortogram using carbon dioxide as contrast agent demonstrates severe focal ostial stenosis of the right renal artery.



