Although epidemiologic studies have shown the impact of height on occurrence and/or prognosis of cardiovascular diseases, the underlying mechanism is unclear. In addition, the relation in patients with ST-segment elevation myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention (PCI) remains unknown. We sought to assess the influence of height on outcomes of patients with acute STEMI undergoing primary PCI and to provide a pathophysiological explanation. All 1,490 patients with STEMI undergoing primary PCI were analyzed. Major adverse cardiac and cerebrovascular events (MACCE) were defined as all-cause mortality, nonfatal myocardial infarction, nonfatal stroke, and unplanned hospitalization for heart failure (HF). Patients were divided into (1) MACCE (+) versus MACCE (−) and (2) first- to third-tertile groups according to height. MACCE (+) group was shorter than MACCE (−) group (164 ± 8 vs 166 ± 8 cm, p = 0.012). Prognostic impact of short stature was significant in older (≥70 years) male patients even after adjusting for co-morbidities (hazard ratio 0.951, 95% confidence interval 0.912 to 0.991, p = 0.017). The first-tertile group showed the worst MACCE-free survival (p = 0.035), and most cases of MACCE were HF (n, 17 [3%] vs 6 [1%] vs 2 [0%], p = 0.004). On post-PCI echocardiography, left atrial volume and early diastolic mitral velocity to early diastolic mitral annulus velocity ratio showed an inverse relation with height (p <0.001 for all) despite similar left ventricular ejection fraction. In conclusion, short stature is associated with occurrence of HF after primary PCI for STEMI, and its influence is prominent in aged male patients presumably for its correlation with diastolic dysfunction.
Even after successful reperfusion with primary percutaneous coronary intervention (PCI), patients with acute ST-segment elevation myocardial infarction (STEMI) are at risk for future major adverse cardiac and cerebrovascular events (MACCE). There have been repeated epidemiologic observations on the impact of height on occurrence and/or outcomes of various cardiovascular (CV) diseases ; however, the association between height and clinical course of patients with STEMI remains to be determined. In addition, the underlying mechanism for the intriguing phenomenon is still inconclusive. In this study, we sought to (1) assess the influence of height on the clinical course of patients undergoing primary PCI for acute STEMI and (2) provide a plausible pathophysiological explanation.
Methods
In this retrospective study, we used data from the “INcheon-Bucheon cohorT of patients undERgoing primary PCI for acute ST-ELevation myocardiaL infARction (INTERSTELLAR) registry.” The INTERSTELLAR registry is a 4-regional hospital-based initiative designed to track the outcomes after primary PCI for acute STEMI in cities of Incheon and Bucheon located in the midwestern part of the Korean peninsula. Patients diagnosed with acute STEMI and treated with primary PCI from 2007 to 2015 were consecutively registered. Diagnosis of acute STEMI was based on clinical data findings including typical symptoms, 12-lead electrocardiography, and blood test. Decisions regarding primary PCI were made by at least 2 attending cardiologists at the time of presentation. Patients previously diagnosed with coronary artery disease, cardiomyopathy, valvulopathy (≥moderate), pericardial disease, and congenital heart disease were excluded. The study protocol was approved by the institutional review board, and we complied with the Declaration of Helsinki (sixth revision). All study subjects provided written informed consents.
Patients were premedicated with aspirin (at least 100 mg). A loading dose of P2Y12 receptor antagonist was administered before PCI. Heparin was administered throughout the procedure to maintain an activated clotting time of ≥250 seconds. A glycoprotein IIb/IIIa receptor blocker was administered at the discretion of the operator. Coronary angiography was performed using standard techniques. Thrombectomy devices, intravascular ultrasound, an intra-aortic balloon pump, and percutaneous cardiopulmonary support were used at the operators’ discretion. Intervention in non–infarct-related arteries during the initial procedure was discouraged. Final angiography was performed to obtain views similar to those obtained before the procedure. Procedural success was defined as no laboratory deaths, no emergency bypass surgery, and Thrombolysis In Myocardial Infarction (TIMI) 3 flow in the distal part of the infarct-related artery with a residual stenosis of <30%.
Transthoracic 2-dimensional echocardiography following the guidelines of the American Society of Echocardiography was performed within 12 hours after the index PCI. Left ventricular (LV) end-diastolic dimension (LVEDD) was measured, and the LV ejection fraction (EF) was calculated using the modified Simpson method. Left atrial (LA) volume was determined using the biplane area-length method and was indexed to the body surface area (LA volume index [LAVI]). From the apical window, a 1- to 2- mm pulsed Doppler sample volume was placed at the mitral valve tip, and mitral flow velocities from 5 to 10 cardiac cycles were recorded. The mitral inflow velocities were traced, and peak velocity of early diastolic filling (E) was determined. Early diastolic mitral annulus velocity (E′) was measured by Doppler tissue imaging at the septal corner of the mitral annulus. For estimation of LV filling pressures, the ratio of E/E′ was calculated. All measurements were repeated 3 times (5 times in case of atrial fibrillation) and averaged. Analysis of echocardiographic data was performed by 2 blinded echocardiographer cardiologists.
After primary PCI, all patients were monitored in the coronary care unit for at least for 24 hours. Standard medical management was provided, and after discharge, the clinical courses of the patients were monitored by cardiologists within 1 month and at 3-month intervals after that. MACCE was designated as all-cause mortality, nonfatal myocardial infarction, nonfatal stroke, and unplanned hospitalization for heart failure (HF). For assessment of the outcomes, all medical records were reviewed, and in cases of loss to follow-up, families of the patients were contacted by telephone.
Continuous data are expressed as a mean ± standard deviation. The baseline characteristics were compared using the 2-sample t test for continuous variables and the chi-square test and Fisher’s exact test for categorical variables. For intertertile comparison, the one-way analysis of variance test was used, and post hoc analyses were performed using the Bonferroni procedure. Pearson bivariate correlation analysis was used for determination of correlation between variables. Cox multiple regression analysis was performed for quantification of the relation between time to death and each potential risk factor. The Statistical Package for the Social Sciences (SPSS 18.0, SPSS Inc., Chicago, Illinois) was used, and p value <0.05 was considered significant.
Results
Baseline characteristics are displayed in Table 1 . During the monitoring period (734 ± 560 days, range 0 to 2,322 days), MACCE occurred in 170 patients (11%) (time to MACCE: 198 ± 366 days, range 0 to 1,439 days). Of note, the MACCE (+) group was significantly shorter, with lower body weight and body mass index than the MACCE (−) group. In the MACCE (+) group, prevalence of multivessel disease and left main artery disease was more common ( Table 2 ). After the procedure, achieving grade 3 TIMI flow and procedural success rate were less common in the MACCE (+) group. In post-PCI echocardiography, the MACCE (+) group had significantly lower LV EF, along with larger LVEDD. Of note, LAVI were larger, and E/E′ were higher in the MACCE (+) group.
| Variables | All (n=1,490) | MACCE | p-value | |
|---|---|---|---|---|
| NO (n=1,320) | YES (n=170) | |||
| Age (years) | 61±13 | 60±13 | 68±13 | <0.001 |
| Men | 1182 (79%) | 1053 (80%) | 129 (76%) | 0.268 |
| Height (cm) | 166±8 | 166±8 | 164±8 | 0.012 |
| Weight (kg) | 66±12 | 67±12 | 63±12 | <0.001 |
| Body mass index (kg/m 2 ) | 24±3 | 25±3 | 24±4 | 0.003 |
| Diabetes mellitus | 404 (27%) | 335 (25%) | 69 (41%) | <0.001 |
| Hypertension | 726 (49%) | 622 (47%) | 104 (61%) | 0.001 |
| Systolic blood pressure (mm Hg) | 125±29 | 126±28 | 111±31 | <0.001 |
| Diastolic blood pressure (mm Hg) | 76±18 | 77±20 | 68±20 | <0.001 |
| Hemoglobin (g/dL) | 14±2 | 14±2 | 13±2 | <0.001 |
| Sodium (mEq/L) | 139±4 | 139±4 | 138±5 | 0.001 |
| Potassium (mEq/L) | 4.0±0.6 | 4.0±0.5 | 4.2±0.8 | <0.001 |
| Glucose (mg/dL) | 178±86 | 173±78 | 214±128 | <0.001 |
| Blood urea nitrogen (mg/dL) | 18±10 | 17±8 | 25±15 | <0.001 |
| Creatinine (mg/dL) | 1.2±1.1 | 1.1±1.1 | 1.6±1.4 | <0.001 |
| Total cholesterol (mg/dL) | 159±62 | 160±63 | 143±52 | 0.048 |
| Low density lipoprotein-cholesterol (mg/dL) | 96±39 | 98±39 | 88±39 | 0.178 |
| High density lipoprotein-cholesterol (mg/dL) | 42±11 | 43±11 | 39±12 | 0.061 |
| Triglyceride (mg/dL) | 133±116 | 134±118 | 112±74 | 0.216 |
| Creatine kinase-myoglobulin (ng/mL) | 49±191 | 47±199 | 70±114 | 0.131 |
| Troponin-I (ng/mL) | 10.1±35.5 | 9.9±36.5 | 11.6±25.9 | 0.604 |
| Variables | All (n=1,490) | MACCE | p-value | |
|---|---|---|---|---|
| NO (n=1,320) | YES (n=170) | |||
| Number of coronary arteries narrowed: | <0.001 | |||
| 1 | 596 (40%) | 554 (42%) | 42 (30%) | |
| 2 | 492 (33%) | 440 (33%) | 52 (31%) | |
| 3 | 402 (27%) | 335 (25%) | 67 (39%) | |
| Infart-related coronary artery | <0.001 | |||
| Left main | 18 (1%) | 8 (1%) | 10 (6%) | |
| Left anterior descending | 758 (51%) | 660 (50%) | 98 (58%) | |
| Left circumflex | 158 (11%) | 144 (11%) | 14 (8%) | |
| Right coronary | 558 (37%) | 508 (38%) | 50 (28%) | |
| Final TIMI flow grade | <0.001 | |||
| 0 | 8 (1%) | 4 (0%) | 4 (2)% | |
| 1 | 15 (1%) | 11 (1%) | 4 (2%) | |
| 2 | 158 (11%) | 120 (9%) | 38 (27%) | |
| 3 | 1309 (87%) | 1185 (90%) | 124 (79%) | |
| Procedural success | 1266 (85%) | 1166 (88%) | 100 (59%) | <0.001 |
| Echocardiography | ||||
| LV EF (%) | 48±12 | 50±11 | 37±15 | <0.001 |
| LVEDD (mm) | 50±5 | 50±5 | 51±7 | 0.010 |
| LVEDD index (mm/m 2 ) | 29±4 | 29±4 | 31±6 | <0.001 |
| LAVI (ml/m 2 ) | 20±9 | 20±8 | 24±14 | 0.014 |
| E/E’ | 13±5 | 12±5 | 16±7 | <0.001 |
As shown in Figure 1 , the first-tertile group (the shortest) showed significantly worse MACCE-free survival than the second-and third-tertile groups. In general, subjects in the first-tertile group suffered from significantly more MACCE ( Table 3 ). Notably, unplanned admission for HF occurred predominantly in the first-tertile group.
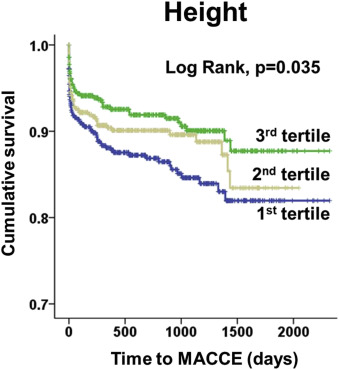
| Variables | 1 st tertile (n=497) | 2 nd tertile (n=498) | 3 rd tertile (n=495) | p-value |
|---|---|---|---|---|
| MACCE | 72 (15%) | 45 (9%) | 52 (11%) | 0.049 |
| Mortality | 48 (10%) | 30 (6%) | 38 (8%) | 0.169 |
| Non-fatal myocardial infarction | 4 (1%) | 7 (1%) | 9 (2%) | 0.210 |
| Heart failure requiring admission | 17 (3%) | 6 (1%) | 2 (0%) | 0.004 |
| Non-fatal stroke | 3 (1%) | 2 (0%) | 3 (1%) | 0.513 |
Because height is naturally influenced by age and gender, our cohort was divided into 4 subgroups (male vs female; aged <70 vs ≥70 years). Multivariate Cox regression analysis results are provided in Table 4 . Interestingly, height predicted MACCE independently only in the older male group. Figure 2 and Figure 3 showed an association between height and LV systolic/diastolic functional parameters and age. LV EF was grossly similar among 3 groups ( Figure 2 ). However, LAVI was the largest ( Figure 2 ) and E/E′ was the highest in the first-tertile group. Similarly, height showed no correlation with LV EF ( Figure 3 ), but there was a significant inverse relation between stature and E/E’ ( Figure 3 ). As expected, height also decreased with age ( Figure 3 ).



