Chapter 30 Radionuclide Imaging in Heart Failure
INTRODUCTION
The epidemic of heart failure affects 5 million people in the United States, with 500,000 new diagnoses made every year.1 Physicians treating heart failure seek answers to several important clinical questions that impact on management:
ESTABLISHED USES OF RADIONUCLIDE IMAGING IN HEART FAILURE
Determining Heart Failure Etiology
Data from epidemiologic studies and clinical trials suggest that 60% to 70% of patients with heart failure have coronary artery disease (CAD). These data underscore the fact that CAD has now overtaken hypertension as the most common cause of heart failure. In a review of 13 randomized, multicenter clinical trials of patients with established heart failure reported in the New England Journal of Medicine, Gheorghiade and Bonow reported that 68% of patients had CAD.2 It is likely that these data underestimate the true prevalence of CAD in heart failure, since patients presenting with LV dysfunction due to acute MI were generally excluded from these trials. It is also important to note that the majority of these reports pertained to patients with chronic heart failure. However, adjudicating etiology is most relevant in patients with new-onset heart failure in whom the detection of significant CAD has important therapeutic and prognostic implications.3
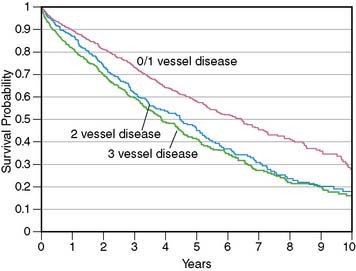
More important, in these clinical trials, an “ischemic” etiology of heart failure was generally attributed to patients with any significant epicardial CAD (usually defined as = 50% diameter stenosis). However, in patients with heart failure and limited-extent CAD (e.g., single-vessel disease) and severe LV dysfunction, the underlying pathophysiology is unlikely to be ischemic LV dysfunction but rather cardiomyopathy of other etiologies coexisting with minor CAD. This distinction between heart failure patients with LV dysfunction resulting from extensive CAD and those with nonischemic LV dysfunction coexisting with limited-extent CAD is prognostically important, as was demonstrated by Felker and colleagues, who followed up 1921 symptomatic heart failure patients enrolled in the Duke database.4 Patients were categorized as having ischemic and nonischemic cardiomyopathy based on two separate definitions. In the traditional definition, heart failure patients with any epicardial CAD equal to 75% were classified as having ischemic cardiomyopathy. In the modified definition, an ischemic etiology was attributed only to patients with extensive CAD, defined as significant left main or proximal left anterior descending coronary artery stenosis, three-vessel disease, or single-vessel disease with prior MI or coronary revascularization, that is, patients with extensive CAD more likely to be etiologically related to heart failure. Therefore, in the modified definition, heart failure patients with single-vessel disease without prior MI or coronary revascularization were categorized as having nonischemic cardiomyopathy. At the end of a median follow-up period of 3.1 years (interquartile range 1.0 to 6.2 years), the authors reported that the modified definition of ischemic cardiomyopathy was a more powerful discriminator of prognosis, and the prognosis of heart failure patients with single-vessel CAD without prior MI or coronary revascularization was identical to that of heart failure patients without CAD (Fig. 30-1). This important observation indicates that the standard definition of ischemic cardiomyopathy applied to most heart failure studies (≥50% diameter stenosis in any coronary artery) may be inadequate for the characterization of heart failure etiology and risk, since it does not enable a distinction between extensive CAD that is likely to be the etiology of heart failure and limited-extent CAD coexisting with heart failure of other etiologies. Making this distinction is a critical step in the evaluation of heart failure patients, since the presence of extensive CAD implies potential benefit from coronary revascularization.5
Current practice guidelines mandate coronary angiography in heart failure patients with angina.6 However, up to 60% of patients with ischemic LV dysfunction do not have angina,7 and the guidelines do not offer firm recommendations for the optimal initial evaluation strategy of these patients.6,8 Given this lack of a unified approach and the potential adverse consequences of not identifying extensive CAD, most heart failure patients are likely to undergo at least one coronary angiogram to exclude CAD, especially after the initial onset of symptoms. Many patients end up having repeat coronary angiograms when clinical symptoms worsen or left ventricular ejection fraction (LVEF) deteriorates.
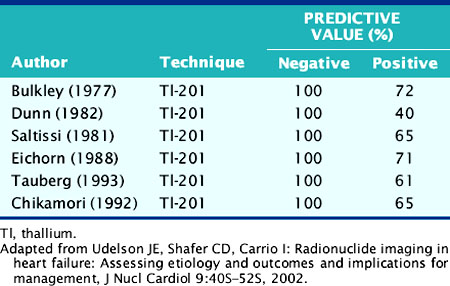
The role of myocardial perfusion imaging (MPI) to diagnose CAD noninvasively in patients with heart failure has been investigated.9 Many of these studies predated contemporary MPI methodology, for example, using nongated, planar thallium-(201Tl) scintigraphy, and yet showed a near-perfect sensitivity and negative predictive value (NPV) (Table 30-1). A more recent study using gated technetium-99m (99mTc) sestamibi single-photon emission computed tomographic (SPECT) MPI incorporating information on both perfusion and regional LV function demonstrated a high sensitivity of 94% but specificity of only 32%.10 Thus, these studies performed in patients with chronic heart failure suggest that MPI may be used to exclude significant CAD in heart failure patients with a high degree of accuracy, but because of the poor specificity of the technique, many heart failure patients without CAD will undergo coronary angiography based on falsely positive MPI results.
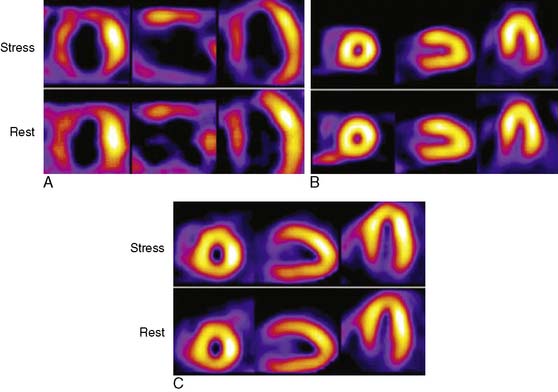
The Investigation of Myocardial Gated SPECT Imaging as Initial Strategy (IMAGING) in Heart Failure study prospectively explored the role of MPI in 201 patients presenting with new-onset heart failure, recruited from 14 hospitals in the United States and United Kingdom.11 Patients underwent exercise or pharmacologic stress MPI with 99mTc sestamibi gated SPECT imaging during or within 2 weeks of the index hospitalization for heart failure. The diagnosis of heart failure was based on the Framingham criteria.12 A subset of patients (37%) underwent coronary angiography based on clinical indications, and the performance characteristics of MPI for CAD diagnosis were derived from this angiographic cohort. A positive MPI was defined by a summed stress score greater than 3 (the sum of the individual segmental perfusion scores on stress MPI, using a 17-segment LV model and a 5-point semiquantitative perfusion score ranging from 0 = normal to 4 = absent perfusion), as validated previously.13 For the diagnosis of any angiographic CAD (= 70% diameter stenosis, prevalence 51%), the sensitivity, specificity, positive predictive value (PPV), and NPV of MPI were 82%, 57%, 67%, and 75%, respectively. For the diagnosis of more extensive CAD, indicating the presence of ischemic LV dysfunction (= 70% stenosis in the left main or proximal left anterior descending coronary artery, = 70% stenosis in two or more major epicardial coronary arteries, or any stenosis = 70% with prior MI or coronary revascularization, prevalence 36%), the corresponding values were 96%, 56%, 55%, and 95%, respectively (Table 30-2). Thus, the principal finding of this study in patients with new-onset heart failure was concordant with prior studies in patients with established heart failure in that MPI has a high NPV for excluding an ischemic etiology for LV dysfunction. Several limitations of this study should be noted. Although prospective and the first study to explore MPI in patients with new-onset heart failure, this was an observational cohort of nonconsecutive patients. Furthermore, coronary angiography was performed in only 37% of patients and was driven by clinical indications, including in some cases, the results of the MPI. Therefore the results were subject to verification bias, which is known to falsely lower specificity and overestimate sensitivity. Selection bias may have also been operative in patients with new-onset heart failure and known CAD, who are sometimes referred directly for coronary angiography. Thus, the applicability of these results to all patients with new-onset heart failure needs further confirmation. Nevertheless, this study in patients with new-onset heart failure, and prior studies in patients with chronic disease, indicate that heart failure patients with a normal MPI are highly unlikely to have significant, etiologically related CAD. Using MPI as an early investigative strategy might avoid the routine use of coronary angiography in some patients. Figure 30-2 shows characteristic patterns of perfusion and function in heart failure patients. Patients with cardiomyopathy of nonischemic etiologies generally have normal or near-normal perfusion. In these patients, coronary angiography can be safely avoided.
Table 30-2 Performance Characteristics of Gated SPECT 99mTc Sestamibi for CAD Diagnosis in Patients with New-Onset Heart Failure from the IMAGING in Heart Failure Study103
| CAD definition | Any CAD: ≥70% stenosis in any coronary artery | Extensive CAD: stenosis ≥ 70% in the LM or proximal LAD, ≥ 70% in ≥ two major epicardial coronary arteries, or any stenosis ≥ 70% with a prior MI or coronary revascularization |
| CAD prevalence by angiography | 51% (n = 38) | 36% (n = 27) |
| Sensitivity % (95% CI) | 82 (66-92) | 96 (81-99) |
| Specificity % (95% CI) | 57 (40-72) | 56 (41-71) |
| PPV % | 67 | 55 |
| NPV % | 75 | 96 |
Criteria for positive SPECT was summed stress score > 3.
CAD, coronary artery disease; LAD, left anterior descending coronary artery; LM, left main coronary artery; MPI, myocardial perfusion imaging; NPV, negative predictive value; PPV, positive predictive value; SPECT, single-photon emission computed tomography; Tc, technetium.
From Soman P, Lahiri A, Mieres JH, et al: Etiology and pathophysiology of new-onset heart failure: Evaluation by myocardial perfusion imaging, J Nucl Cardiol 16:82?91, 2009.
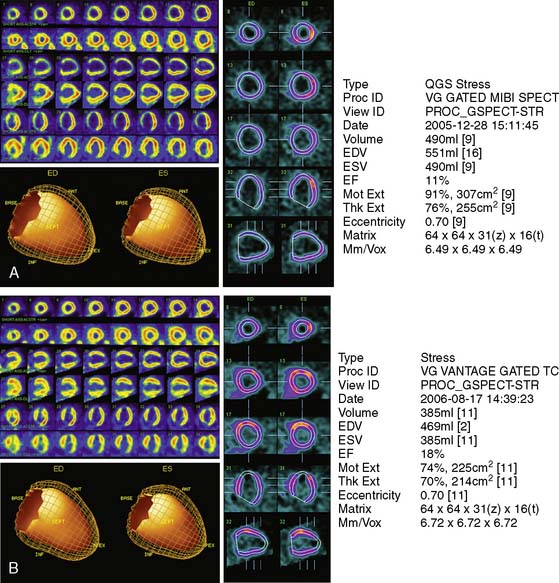
The predominant cause of false-positive MI is soft-tissue attenuation, although pathophysiologic phenomena such as myocardial fibrosis and endothelial dysfunction produce abnormal MPI in some patients with unobstructed epicardial coronary arteries.14,15 Although quantitative attenuation-correction techniques have been shown in clinical trials to improve diagnostic specificity of SPECT MPI, this approach has not been tested specifically in patients with heart failure.16 Given the propensity of attenuation artifacts in patients with a dilated LV, attenuation correction is likely to favorably impact specificity in this population. Figure 30-3 shows the gated SPECT images from a patient in whom the attenuation correction formed using a gadolinium-153 transmission scan was useful in correctly identified nonischemic LV dysfunction.
With the advent of cardiac CT, small, single-center studies have explored the utility of coronary calcium scoring and CT coronary angiography for the diagnosis of CAD in heart failure patients.17,18 Like MPI, coronary calcium scoring and CT coronary angiography have good NPV for this application. In the future, combined SPECT/CT imaging may improve diagnostic accuracy.
Measuring Left Ventricular (Dys)Function (See Chapters 12 and 13)
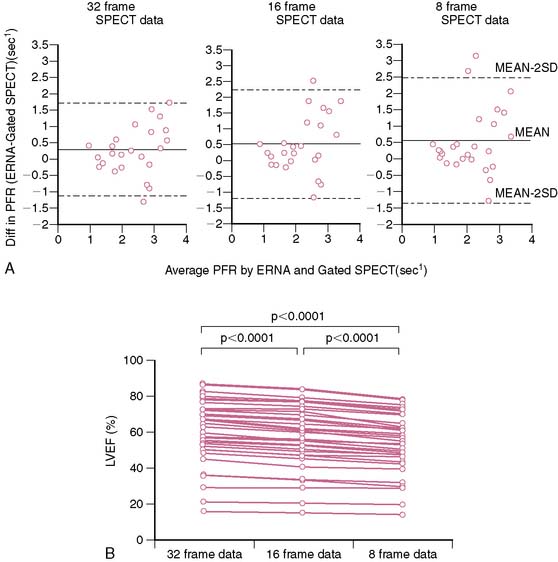
Radionuclide techniques are well validated and widely applied for the assessment of LV function. While radionuclide ventriculography (RVG) was the traditional radionuclide technique used to determine LV systolic and diastolic function, this method has been largely supplanted by gated SPECT imaging, which is extensively used for myocardial perfusion assessment and provides simultaneous information on LV systolic function. Sixteen-frame ECG gating provides an accurate assessment of LV systolic function, but it may not provide a sufficiently high-fidelity time-volume curve for diastolic function assessment (Fig. 30-4).19 Assessment of diastolic function can be performed with 32- or 64-frame gating, which can be impractical for routine MPI because of the long scanning time required for collection of adequate counts.19,20
Assessment of ejection fraction by gated SPECT imaging has been validated against other established imaging modalities, including echocardiography, radionuclide ventriculography, and newer techniques with high spatial resolution, such as magnetic resonance imaging, with excellent correlation.20 However, it must be noted that the normal limits of ejection fraction vary depending on the modality used.21,22 This intermodality variability is exaggerated in patients with LV systolic dysfunction,22 an observation that has been overlooked even in the design of major clinical trials requiring precise measurement of LV function, such as the implantable cardioverter-defibrillator trials in heart failure patients. While these trials recruited patients based on specific ejection fraction criteria, a modality of ejection fraction measurement was usually not specified.23,24 Normal limits also vary based on gated SPECT methodology, with 8-frame gating resulting in lower ejection fraction values that correlate less well with RVG compared to 16-frame gating19,25 and with the software used for quantification of ejection fraction and volumes (see Fig. 30-4).26 In some laboratories including the author’s, the lower limit of normality for ejection fraction measurement by gated SPECT is set variably depending on the frame rate used for gating (45% and 50% for 8-frame and 16-frame gating, respectively). Compared to two-dimensional echocardiography, which is also widely used for assessment of LV systolic function, a significant advantage of gated SPECT estimation of LV systolic function is its fully automated application, resulting in high reproducibility. Studies indicate that the variability in serial estimations of LV ejection fraction from gated rest 99mTc SPECT scans is approximately ±5% (Fig. 30-5).27,28 Serial gated SPECT imaging can be effectively used to assess changes in perfusion and function following therapeutic interventions (Fig. 30-6A and B).
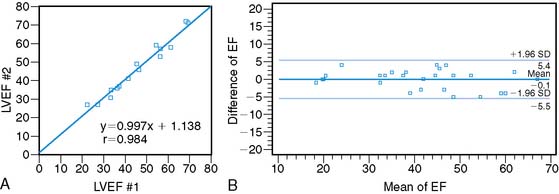
Figure 30-5 Reproducibility of left ventricular ejection fraction (LVEF) on serial resting gated SPECT imaging. A, Data from Johnson et al.27 on 15 patients who underwent serial rest thallium-201 imaging. LVEF obtained from the first and second studies are plotted on the x– and y-axes, respectively. The regression line is not significantly different from the line of identity, indicating close correlation between the two measurements. B, Data from Hyun et al.28 on 26 patients, showing that the 2-SD limits of difference in two serial measurements of LVEF by technetium-99m gated SPECT imaging assessed by Bland-Altman plotting was ± 5.5%. In this study, the variability between serial gated SPECT thallium-201 imaging was higher.
Predicting Benefit from Coronary Revascularization: Assessment of Myocardial Viability (See Chapters 37 and 38)
In patients with ischemic LV dysfunction, the assessment of myocardial viability is a critical step in management planning. On one hand, patients with severe LV systolic dysfunction undergoing coronary bypass grafting are at high risk for perioperative mortality.29 On the other, the presence of significant amounts of residual myocardial viability portends the potential for improvement in symptoms,30–32 regional LV function,33,34 global LV function,35,36 and prognosis5,37 following coronary revascularization. It is important to note that while the use of functional recovery is a convenient standard for viability studies, it may not capture the full benefit of revascularization, which may confer other important benefits such as attenuation of progressive remodeling and prevention of arrhythmia independently of functional recovery.38–40 LV function may also continue to improve for several months following revascularization, so a single assessment, if not timed appropriately, may underestimate the full extent of functional recovery.39 Finally, resting regional function is primarily determined by endocardial thickening, which is unlikely to improve in patients who have suffered a subendocardial MI despite preserved myocardial viability.41–43 A more important criterion for assessing the usefulness of noninvasive viability testing should be whether it can drive therapeutic decisions that ultimately improve patient outcome. While the field of viability testing is somewhat limited by the lack of randomized control studies, a meta-analysis by Allman and colleagues5 of 24 studies of viability testing with MPI or dobutamine echocardiography that reported on survival after coronary revascularization provides important insights. In patients with predominantly viable myocardium, follow-up on medical therapy was associated with a 16% annual mortality. Similar patients who were revascularized experienced an annual mortality of only 3.2%—that is, an 80% reduction in mortality. In contrast, the choice of medical therapy or revascularization had no significant impact on annual mortality (7.7% versus 6.2%, respectively) in patients with predominantly nonviable myocardium. Despite the known limitations of pooling data from observational cohort studies, which can bring into play selection biases that cannot be evaluated when meta-analyzing published literature, these data provide a strong signal that the presence of residual myocardial viability in patients with LV systolic dysfunction is a marker of very high risk without revascularization.
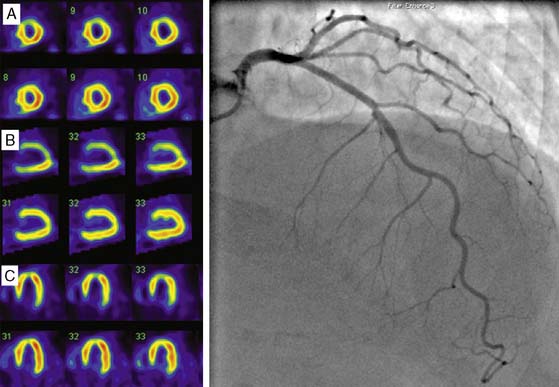
Clinical studies suggest that residual myocardial viability is prevalent among patients with ischemic LV dysfunction.44,45 For example, in the CHRISTMAS trial of 489 patients with chronic heart failure due to ischemic LV dysfunction (LVEF 29 ± 11%), 79% had viable myocardium demonstrated by 99mTc sestamibi SPECT imaging.45 It must be noted that viability exists as a continuum, ranging from patients with extensive scar tissue and minimal residual viability, to those with minimal scar tissue and predominantly viable but dysfunctional myocardium due to varying combinations of hibernating and repetitive stunning.46,47 Patient outcome is dependent on not only the presence but also the extent of viability, and a critical threshold mass of viable myocardium may be necessary for functional recovery and prognostic benefit to occur from revascularization.48 Therefore, while several clinical and laboratory parameters, including anginal symptoms, absence of Q waves on the electrocardiogram, and absence of thinning and akinesis on echocardiography, indicate the presence of some viability, a systematic assessment of the degree and extent of viability is often indicated for management planning and prognostication. Techniques used for viability assessment are based on the demonstration of preserved myocyte metabolism (F-18 fluorodeoxyglucose positron emission tomography [PET]), cellular integrity (SPECT with 201Tl or 99mTc ligands), contractile reserve (dobutamine echocardiography), microvascular integrity (myocardial contrast echocardiography), or the absence of scar tissue (gadolinium-enhanced cardiac magnetic resonance [CMR] imaging). The use of PET and SPECT for viability assessment is backed by a substantial literature, with SPECT having the advantages of wider availability and relative ease of use, and PET having a slightly higher overall accuracy.33 The property of redistribution confers an advantage to 201Tl compared with 99mTc agents for viability assessment in dysfunctional myocardial segments supplied by a critically stenosed coronary artery. Here the continued uptake of 201Tl over time results in higher tracer uptake on the delayed compared to early scan, thus signaling the presence of viable myocardium (Fig. 30-7).49,50 However, when used with nitrate enhancement and quantitative interpretation, 99mTc-sestamibi SPECT has comparable accuracy for viability assessment.51,52 When compared with other imaging modalities for viability assessment, radionuclide techniques have comparable predictive accuracy for functional recovery.33 Comparative studies also indicate that slight differences in sensitivity and specificity among these modalities may not impact on management decision making in a clinically meaningful way.53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree