Chapter 9 Digital/Fast SPECT
Systems and Software
INTRODUCTION
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) has become a widely used and well-established medical imaging test. However, it suffers from some fundamental limitations that include long image acquisition, low image resolution, and patient radiation dose. Scan time is generally on the order of 15 to 20 minutes for each stress and rest acquisition, resulting in long overall test times and frequent artifacts from patient motion during the scan. Reduction of imaging times has been long recognized as an important factor in reducing patient motion artifacts and increasing throughput. Various efforts have been taken to reduce this time with standard equipment and cameras.1–3 Furthermore, the recent increase in the use of coronary computed tomography angiography (CCTA) in patients who also have SPECT MPI studies has intensified the concern about the levels of radiation associated with SPECT.4 Some of these limitations are intrinsically linked to each other; longer acquisition times could be used with lower injected doses, and higher doses could be used to shorten acquisition times.
DEDICATED CARDIAC IMAGING SYSTEMS
Several new dedicated hardware camera systems that have been introduced by various vendors for cardiac imaging attempt to improve imaging parameters primarily by optimizing acquisition geometry, collimator design, and reconstruction software.5 Innovative designs of the gantry and detectors have been introduced that allow increased sampling of the myocardial region and thus allow better local sensitivity for MPI imaging. The primary goals for the development of these systems are twofold: (1) to achieve improved spatial resolution and sensitivity and therefore allow for faster imaging times and (2) to improve patient comfort by permitting imaging in an upright or reclining position, eliminating the need to position the patient’s arms above the head. Furthermore, claustrophobic effects have been reduced or eliminated by technologies that reduce the size of the detector geometries and the associated mechanical structures. Overall sizes of the systems have also been reduced so they can be placed in locations with minimal available floor space. As a consequence of faster imaging times and more comfortable patient positioning, these systems have the additional benefit of reducing patient motion during a scan. In this section, we describe various available hardware designs and related reconstruction techniques.
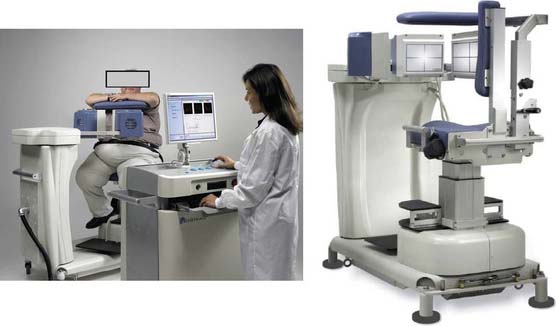
Digirad Cardius 3 XPO
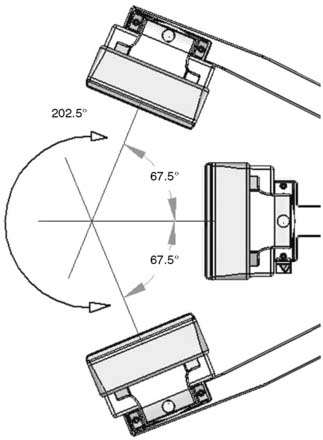
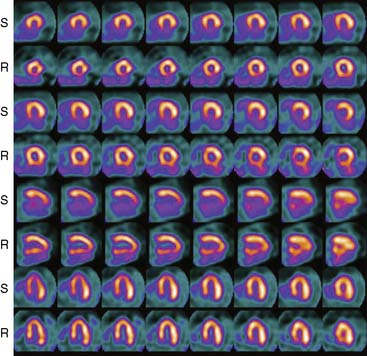
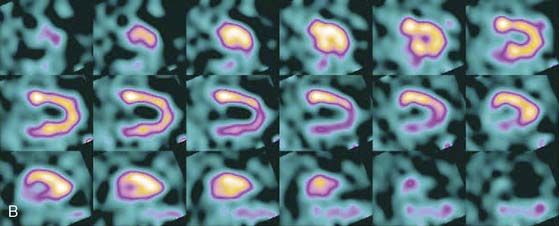
The first of these systems to be introduced was the Cardius XPO line manufactured by Digirad Corporation (Poway, CA). Although the initial design of a similar Digirad system was described in 1998 with the use of cadmium zinc telluride (CdZnTe, or CZT),6 all manufactured systems use solid-state cesium iodide (CsI)-photodiode detectors. This system can be configured in 1-, 2-, or 3-detector configurations.7 The Cardius 3 XPO is shown in Figure 9-1. Current models make use of pixilated CsI(Tl) detectors and photodiodes to configure detector heads that are more compact than conventional cameras equipped with photomultipliers. Each detector head is 21.2 × 15.8 cm and contains an array of 768 6.1 × 6.1 × 5 mm-thick CsI(Tl) crystals coupled to individual silicon photodiodes that are used to convert the light output of the crystals to electrical pulses. Digital software logic is used to process the signals and create images instead of analog Anger positional circuits. In the three-detector system, the detector heads are positioned at 67.5 degrees between heads, as shown in Figure 9-2. Heads are allowed to be moved in and out (closer to or farther away from the patient). For imaging, the patient sits on a chair with his arms placed on an armrest above the detectors. Data acquisition is typically accomplished in 7.5 minutes by rotating the patient chair by 67.5 degrees, producing a total acquisition arc of 202.5 degrees. With this system, the manufacturer reports a reconstructed spatial resolution of 8.95 mm (at a 20-cm orbit radius) and a sensitivity of 234 cpm/μCi, using the system’s cardiac collimator and a three-dimensional (3D) version of the ordered subset expectation maximization (OSEM) approach for reconstruction. Images of a patient with an inferior-wall defect acquired with this system are shown in Figure 9-3. These systems are now used clinically in several sites, and preliminary reports have been published comparing their performance to that of a standard dual-headed camera when it was found that similar quality could be obtained with a 38% reduction in acquisition time.8
Preliminary data have recently shown that the acquisition time can be further reduced with this scanner by the application of optimized image reconstruction protocols developed by Digirad. The nSPEED reconstruction9 models the depth-dependent detector spatial response of the SPECT systems with a 3D version of the OSEM reconstruction method. In preliminary reports,10 the image quality improvement with nSPEED, compared to a conventional two-dimensional OSEM technique,11 enables the reduction of acquisition time by 50% while maintaining image quality and information.
CardiArc
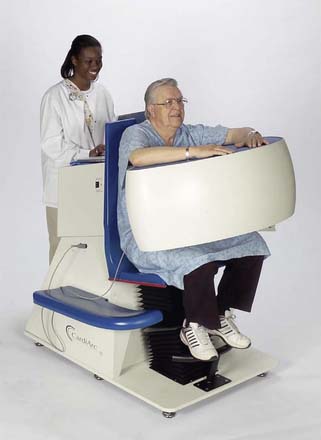
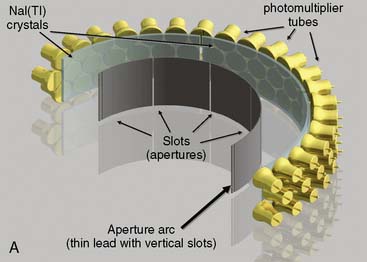
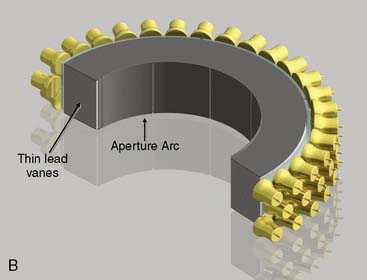
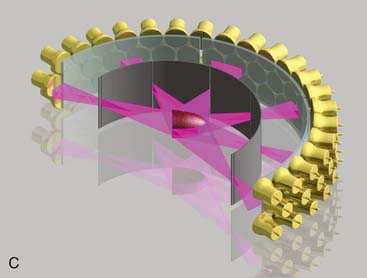
CardiArc Limited (Canton, MI) has developed a dedicated nuclear cardiology SPECT camera in which the detector and collimator are redesigned and optimized specifically for cardiac imaging.12 This device has no visibly moving parts but has a single internally moving part, which is hidden from the patient.13 Therefore, from the outside, the detector appears motionless, and for comfort the patient can be positioned upright. Scan times reported by the company are as short as 2 minutes.12 The camera system and a typical patient position are shown in Figure 9-4. This system was originally designed to use arrays of CZT crystals as detectors. However, owing to the high cost of CZT material and potential long-term stability issues with CZT,14 the detector design was changed to enable commercial production. Figure 9-5 illustrates the design and the principle of operation of the current model. The system incorporates a high-resolution detector with three curved sodium iodide–thallium (NaI[Tl]) crystals with graduated grooving technology and an array of 60 photomultiplier tubes arranged in three rows (see Fig. 9-5A). The gantry uses a proprietary digital process developed by CardiArc that replaces the conventional Anger logic. Horizontal photon collimation in each slice is accomplished using a thin, curved lead sheet with six narrow vertical slots (aperture arc; see Fig. 9-5A). Vertical slice collimation is accomplished using a series of stationary lead vanes that are stacked vertically between the aperture arc and the NaI(Tl) crystals (see Fig. 9-5B). In this way, data are collected as multiple 1 mm–thick slices using the six vertical apertures to collimate photons so they are detected continuously across the detector surface, with no overlap of data from different apertures. During acquisition, the aperture arc rotates to acquire data from multiple projections, providing 1280 angular samples in 0.14-degree increments over 180 degrees, which is an order of magnitude greater than the conventional camera angular sampling (typically 3 degrees). All detector pixels are active simultaneously, and photons can be detected from multiple angles (see Fig. 9-5C). The movement of the aperture arc is synchronized electronically with the areas of the NaI(Tl) crystals that are imaging the photons passing through the individual slots. The aperture arc’s weight of 35 pounds is lighter than traditional moving gantries, which facilities motion control. The aperture arc movement ranges 9 inches to cover the entire cardiac field of view, and each traverse of the arc takes 10 seconds.
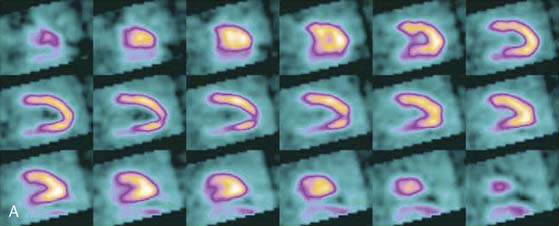
SPECT reconstructed spatial resolution values (full-width half-maximum) quoted by CardiArc range from 3.6 mm (at 82 mm source-to-aperture arc distance) to 7.8 mm (at 337 mm source-to-aperture arc distance). An independent evaluation concluded that the CardiArc system appears to gain image quality by a factor of 5 to 10 when compared to the conventional dual-head camera.15 A comparison of patient imaging capabilities is shown in Figure 9-6.
Spectrum Dynamics
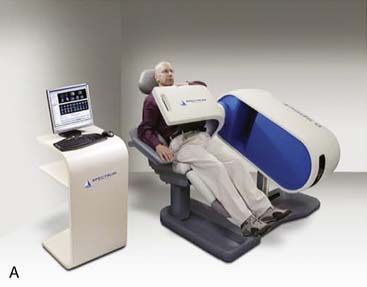
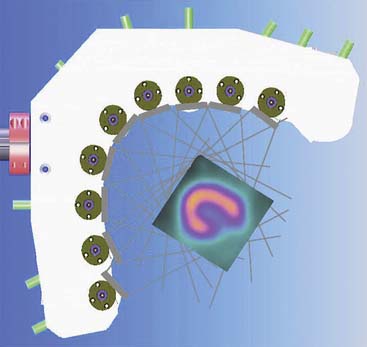
Spectrum Dynamics (Haifa, Israel) has manufactured a system called D-SPECT. The design and principle of its operation are shown in Figure 9-7. The patient is imaged in either a semireclining position with the left arm placed on top of the camera (see Fig. 9-7A) or in the supine position. Acquisition time as short as 2 minutes has been reported.16 This system uses pixilated CZT detector arrays (see Fig. 9-7B) mounted in nine vertical columns and placed in a 90-degree gantry geometry (see Fig. 9-7C). While CZT detectors are higher in cost, they have advantages of superior energy resolution (by a factor of approximately 1.7 at 140 keV) and compact size compared to the combination of NaI(Tl) with photomultiplier tubes of the conventional Anger camera. With D-SPECT, each detector column is fixed in a mechanical mounting, and the data acquisition is performed by rotating these multiple columns in synchrony. The photons from a given location are detected at multiple angles by multiple columns as the fields of view of the detectors are swept through the region of interest. Each column (see Fig. 9-7B) consists of an array of 1024 CZT elements (2.46 × 2.46 × 5 mm thick) arranged in a 16 × 64 element array with an approximate size of 40 × 160 mm. Each column is fitted with square parallel-hole high-sensitivity collimators, such that the dimensions of each hole are matched to the size of a single detector element. The collimators are fabricated from tungsten to eliminate the production of lead x-rays that might interfere with technetium-201 (201Tl) imaging. The collimators have a larger effective diameter than conventional low-energy high-resolution collimators used with scintillation cameras, yielding a significant gain in their geometric efficiency. The collimator has a hole length of 24.5 mm, with a 2.46-mm pitch and 2.26-mm hole diameter. The compensation for the loss in geometric spatial resolution that results from this design is accomplished by the software compensation methods. All data are collected in list mode. A proprietary Broadview iterative reconstruction algorithm based on the maximum-likelihood expectation maximization (MLEM) approach, with resolution recovery and use of the cardiac shape priors, has been developed and patented by the manufacturer.17 It allows the recovery of image resolution to 5 mm in line source experiments.
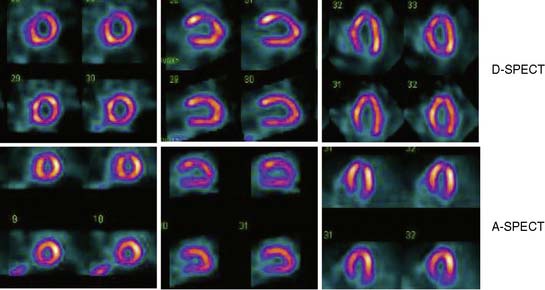
Data acquisition is accomplished in a two-step process. First, a 1-minute pre-scan is performed to identify the location of the region of interest. Scan limits and timings are then set for each detector column, and the final scan is performed with each detector column rotating within the limits set from the pre-scan data. This process is shown diagrammatically in Figure 9-8. This process is termed region-of-interest-centric scanning by the manufacturer, because the scan field is limited to only the myocardial region. It has not been possible to measure an absolute value of sensitivity for this system as prescribed by the National Electrical Manufacturers Association quality-control standards,18 because the sensitivity is significantly dependent on the field of view, defined individually for each patient by the pre-scan process. However, the most centrally located point has been reported to demonstrate a sensitivity of 1407 counts/μCi/min compared to the 160 to 240 counts/μCi/min range generally observed with standard cameras.19 A case example showing image quality on both D-SPECT and A-SPECT, obtained with the same isotope injection, is shown in Figure 9-9. In a recently published study, when D-SPECT was compared to A-SPECT, the myocardial count rate (with the same injection of the isotope) was 7 to 8 times higher for D-SPECT (Fig. 9-10).16 Preliminary work has shown that simultaneous dual-isotope SPECT MPI with this camera is feasible using 201Tl and technetium-99m, taking advantage of the improved energy resolution of CZT.20