Chapter 41 Cardiac Neurotransmission Imaging
Single-Photon Emission Computed Tomography
INTRODUCTION
Afferent tracts arising from myocardial nerve terminals and reflex receptors (e.g., baroreceptors) are integrated centrally within hypothalamic and medullary cardiostimulatory and cardioinhibitory brain centers, and on central modulation of sympathetic and parasympathetic outflow at the level of the spinal cord and within cervical and thoracic ganglia. There are additional levels of intricate processing within the extraspinal cervical and thoracic ganglia and within the cardiac ganglionic plexus, where interneurons provide noncentral integration.1
The SNS is dominant in the heart, principally in the ventricles. Sympathetic nerve fibers travel along the vascular structures, penetrating into the myocardium from the epicardium toward the endocardium. There is a gradient distribution of nerve terminals from the base to the apex of the left ventricle (LV).2
Most of the released NE undergoes reuptake in the presynaptic neurons by the NE transporter (NET), a saturable and sodium-, energy-, and temperature-dependent transport protein, cocaine and desipramine sensitive, with high affinity to catecholamines and catecholamine analogs, in a process known as uptake-1.3 In addition, there is NE uptake by a second, corticosterone- and clonidine-sensitive, low-affinity, high-capacity, extraneuronal transport system known as uptake-2.4 Uptake-1 predominates at low concentrations of catecholamines, whereas uptake-2 predominates at higher concentrations,5 but its contribution in humans is low.6
Once NE is again inside the nerve terminal, it is either metabolized by monoamine oxidase (MAO) or stored in vesicles by the vesicular monoamine transporter (VMAT), a proton-dependent transport protein localized in the vesicle membrane. Neuronal uptake-1 regulates the concentration of adrenergic neurotransmitters in the synaptic cleft, playing important physiologic and pathophysiologic roles in modifying signal transduction and extraneuronal catecholamine concentration. It is of paramount importance in protecting the heart from the deleterious effects of elevated levels of circulating catecholamines.7,8
METAIODOBENZYLGUANIDINE (See Chapter 30)
MIBG is an iodinated aromatic analog of the hypotensive false neurotransmitter, guanethidine, which in its turn, is an analog of NE. MIBG and NE have similar molecular structures, and both utilize the same uptake, storage, and release mechanisms in the sympathetic nerve endings. However, MIBG is neither metabolized at nor interacts with postsynaptic receptors. Owing to these characteristics, the labeling of MIBG with iodine-123 (123I) enables in vivo scintigraphic visualization of the sympathetic postganglionic presynaptic fibers,9–14 thus allowing the assessment of both anatomic integrity12,13 and function of the nerve terminals.13 The ability of sympathetic nerves to take up radiolabeled catecholamines was shown to be a more sensitive indicator of intact neuronal function than cardiac NE content.15
MIBG, like catecholamines, is primarily removed from the circulation by the uptake-1 system. Blocking experiments have shown that uptake-2 is responsible for up to 61% of cardiac MIBG uptake.11–13 For clinical studies, the production of 123I-MIBG involves isotopic exchange, which results in a low specific activity (lower ratio of radiolabeled to nonradiolabeled MIBG), with considerable amount of carrier in the final product. Since uptake-2 predominates at higher concentrations of MIBG,5 scintigraphic images do not improve by increasing the dose of 123I-MIBG, which might even lead to saturation of the uptake-1. Reduction of the total amount of MIBG in combination with a higher specific activity (no-carrier-added MIBG) improves myocardial uptake of MIBG through uptake-1, leading to better contrast between specific and nonspecific MIBG uptake as compared with carrier-added MIBG.16
Planar and SPECT Cardiac Imaging with 123I-MIBG
Before the administration of the radiotracer, it is necessary to withdraw medications known to interfere with the accumulation of MIBG (Table 41-1), taking into account their respective blood half-lives.17
Table 41-1 Drugs Known or Expected to Reduce MIBG Neuronal Accumulation
| Drugs | Mechanism of Interference |
|---|---|
| Tricyclic antidepressants, cocaine, labetalol | Inhibition of uptake-1 |
| Reserpine, tetrabenazine | Inhibition of vesicular uptake |
| Norepinephrine, serotonin, guanethidine | Competition for vesicular uptake |
| Reserpine, guanethidine, labetalol, sympathicomimetic amines (e.g., phenylpropanolamines, anorectics) | Depletion of content from storage vesicles |
| Calcium antagonists | Calcium mediated |
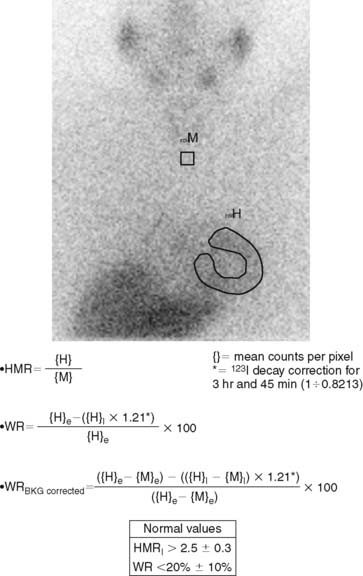
123I-MIBG uptake is semiquantified by calculating a heart-to-mediastinum ratio (HMR)18–20 after drawing regions of interest (ROIs) over the heart (including or not including the cavity) and the upper mediastinum (avoiding the thyroid gland) in the planar anterior view. Average counts per pixel in the myocardium are divided by average counts per pixel in the mediastinum.21 The myocardial washout rate (WR) from initial to late images is also calculated; WR is expressed in percentage as the rate of decrease in myocardial counts over time between early and late imaging (normalized to mediastinal activity) (Fig. 41-1). The late HMR reflects the relative distribution of sympathetic nerve terminals, offering the global information about neuronal function resulting from uptake, storage, and release.22 The WR reflects the neuronal integrity or sympathetic tone, mainly representing the uptake-1.22 More studies are needed to establish the differences in early HMR, late HMR, and WR. Intraobserver and interobserver variabilities of these calculations are less than 5%.21 Normal values for late HMR and WR are ≥ 2.5 ± 0.3 and ≤ 20% ± 10, respectively,23 but vary related to age (late HMR inversely, WR directly).20,24 Moreover, these parameters fluctuate significantly due to lack of validation and standardization of acquisition parameters such as acquisition duration and type of collimation used (in relation to the additional photo peak of 123I at 529 keV, capable of septal penetration when using the common low-energy collimators). Improved standardization of cardiac 123I-MIBG imaging parameters would contribute to increased clinical applicability for this procedure.25
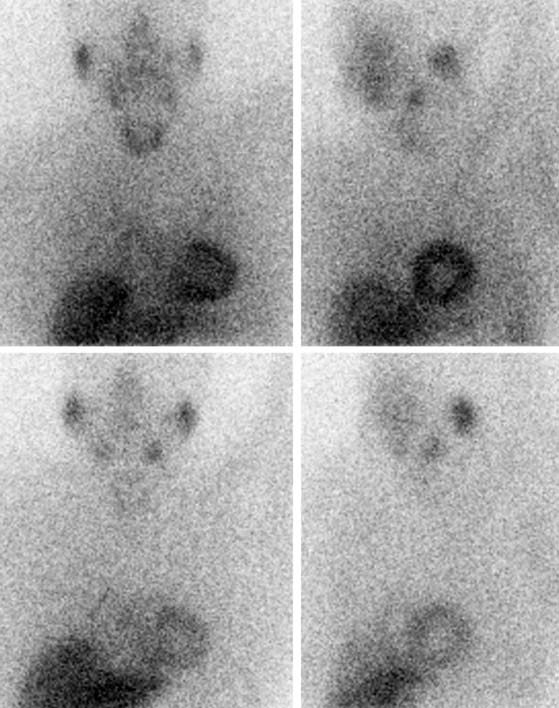
SPECT images can be scored using a point scale for visual evaluation of 123I-MIBG concentration in given cardiac segments, comparable to a myocardial perfusion imaging scoring approach. Careful interpretation should be performed, with knowledge of normal variants and potential artifacts. Normal cardiac 123I-MIBG distribution includes a relatively low uptake in the inferior wall,26 which is more pronounced in the elderly (Figs. 41-2 and 41-3).20 In addition, there may be substantial 123I-MIBG uptake in the liver, which overlaps the inferior LV wall. Moreover, scattering from the lung field to the lateral LV wall may also occur. Polar maps (see Fig. 41-3)27 can be generated from SPECT data and compared with those of normal individuals. Scores of the extension and severity of 123I-MIBG eventual defects and calculation of the mean global and regional WR of the LV are feasible. However, it has to be taken into account that in some pathophysiologic conditions, cardiac 123I-MIBG uptake may be severely reduced, hampering the acquisition and processing of the tomographic slices (Fig. 41-4).
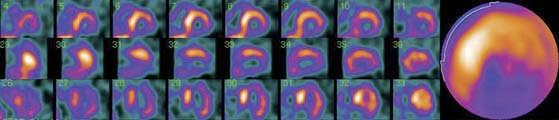
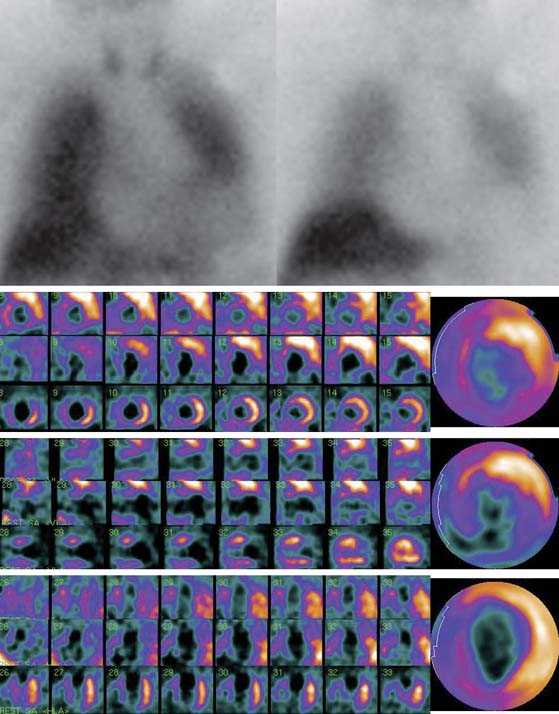
Figure 41-3 Late 123I-MIBG SPECT images of the subject shown in Figure 41-2. Both the SPECT slices and the polar map show reduced tracer uptake in the inferior wall and apex, which is more apparent than on planar images and should be considered a normal variant in the elderly. Upper row: short-axis slices (extending from apex to base). Middle row: vertical long-axis slices (extending from septum to lateral wall). Bottom row: horizontal long-axis slices (extending from inferior wall to anterior wall). 123I-MIBG, iodine-123-labeled metaiodobenzylguanidine; SPECT, single-photon emission computed tomography.
123I-MIBG Imaging in Coronary Artery Disease
The sympathetic nervous tissue is more sensitive to the effects of ischemia than the myocardial tissue.28–30 It has been shown that the uptake of 123I-MIBG is significantly reduced in areas of myocardial infarction (MI)31–33 and adjacent noninfarcted regions (see Fig. 41-4),34,35 as well as in areas with acute and chronic ischemia.36,37 It is likely that ischemia induces damage to sympathetic neurons, which may take a long time to regenerate, and that episodes of ischemia result in decreased 123I-MIBG uptake.38 Gaudino et al.39 provided evidence of good correspondence between 123I-MIBG imaging and the presence or absence of sympathetic cardiac nerves by direct immunohistochemical staining in patients with LV aneurysms due to long-lasting anterior MI.
Reinnervation late after MI in periinfarct regions has been demonstrated by reappearance of 123I-MIBG uptake, which may be in part responsible for the improvement of function. However, reinnervation may be incomplete as late as 3 months after acute MI.40 Hartikainen et al.41 examined 123I-MIBG uptake at 3 and 12 months after a first MI and found no difference in 123I-MIBG activity over time within the infarcted zone but an increase in activity in the peri-infarcted region, without a change in perfusion.
Dissociation between recovery of myocardial perfusion after an ischemic event and myocardial innervation, as determined with 123I-MIBG SPECT, was reported by Matsunari et al.42 Despite considerable myocardial salvage following coronary artery reperfusion, 123I-MIBG images obtained a mean of 11 days after MI and reperfusion demonstrated a persistent area of myocardial denervation within the LV. This area was comparable to the area of ischemic myocardium at risk, as determined by myocardial perfusion SPECT during the acute ischemic event.42 Such modifications of cardiac neuronal function may have an important role in the pathophysiology of HF and arrhythmias, but further studies are warranted to determine the clinical value of innervation imaging in ischemic heart disease.
Concordance between the extent of 123I-MIBG defect at rest and perfusion defect at exercise has been shown in patients with coronary artery disease (CAD).43 This concordance suggests that resting imaging with 123I-MIBG combined with resting myocardial perfusion imaging may be useful as a marker of reversible ischemia in patients incapable of exercise and with contraindications to pharmacologic stress.43 Another potential role for 123I-MIBG imaging at rest is the detection of sporadic transient ischemic attacks such as those of vasospastic angina, otherwise difficult to identify with myocardial perfusion imaging. Watanabe and coworkers44 studied the clinical implications of fatty acid metabolic imaging with iodine-123-labeled β-methyl-iodophenyl pentadecanoic acid (123I-BMIPP) and of cardiac sympathetic nerve functional imaging with 123I-MIBG in patients with vasospastic angina. 123I-BMIPP imaging showed superior specificity but inferior sensitivity compared to 123I-MIBG imaging for the identification of vasospastic regions. The combination of fatty acid and sympathetic imaging was highly accurate for determining the presence and location of vasospasm and superior to the visualization of wall-motion abnormalities by left ventriculography. The mechanism for the decreased 123I-MIBG uptake in coronary vasospastic angina is thought to be memorization of repeated ischemia, but in its turn, the abnormal sympathetic function detected may also be involved in the pathogenesis of the coronary spasms. The property of “ischemic memory” for resting 123I-MIBG imaging also has promise in the evaluation of the area at risk in the subacute phase of acute coronary syndromes by revealing more extensive defects than myocardial perfusion imaging with technetium-labeled agents.45
Although association of 123I-MIBG defects with clinical angina can be explained as a consequence of ischemia, it is intriguing that the perception of pain occurs from the area that lacks sympathetic innervation.34 However, since 123I-MIBG defects in the ischemic territory are not absolute, partial neuronal innervation may allow perception of chest pain.
The number of patients with end-stage ischemic heart disease who cannot be revascularized because of either previous surgery or poor vessels for grafting, angioplasty, or stenting is increasing. When medical therapy fails, these patients are left with disabling angina and/or ischemic HF. New methods to revascularize the heart are under investigation. One method consists of the placement of laser channels into the myocardium using either transmural or endocardial approaches, which would connect cavitary blood with the myocardium to offer nutrient perfusion. Patients treated with either transmural or endocardial laser experience relief of anginal symptoms within days of the procedure, which has been attributed to denervation. Nevertheless, Johnson et al.46 demonstrated that there is negligible regional denervation 3 days after placement of endomyocardial laser channels using autoradiography with 125I-MIBG and hematoxylin-eosin staining in a swine model. Muxi et al,47 studying patients with CAD who underwent transmyocardial laser revascularization, reported significant improvement in stress myocardial perfusion scintigraphy in revascularized areas after 3 and 12 months of the intervention. At 3 months after the procedure, a significant worsening of myocardial innervation was observed in such areas by means of 123I-MIBG, which recovered partially at 12 months. Thus, transmyocardial laser revascularization may involve both perfusion improvement and denervation, mainly at 3 months, the latter partially recovering at 12 months.
123I-MIBG Imaging in Arrhythmogenesis
Cardiovascular disease is the single most common cause of natural death in developed countries. SCD accounts for 50% of all cardiovascular deaths. The most common primary electrical event at the time of SCD is ventricular tachycardia (VT), which degenerates first to ventricular fibrillation (VF) and later to asystole. Bradyarrhythmia or electromechanical dissociation are also frequently documented, mainly in patients with advanced heart disease.48
SCD is usually attributed to structural heart disease, principally in the setting of acute or chronic MI (80%). The cardiomyopathies account for another 10% to 15% of all SCDs. The remaining 5% to 10% of SCDs occur in other cardiac disorders, such as valvular or congenital heart diseases, acquired infiltrative diseases, and ion channelopathies.48 Despite all these epidemiologic data, most SCDs occur among subjects with no previous cardiovascular history, with an apparent need for strategies to allow screening for markers of increased risk of death from arrhythmia among those with low- and intermediate-risk profiles48,49 and better therapy and outcome for these individuals.
Current evidence underscores the importance of the autonomic influences in triggering and sustaining arrhythmias in patients with susceptible substrate,50 and provides important mechanistic insights into the ionic and cellular mechanisms involved.1
Heterogeneity of sympathetic innervation in response to injury is highly arrhythmogenic. Sequential changes consisting of nerve degeneration followed by neurilemma cell proliferation and axonal regeneration have been described after episodes of MI, rapid pacing, radiofrequency ablation, hypercholesterolemia, and stem-cell transplantation.51 According to this “nerve-sprouting” hypothesis, it is possible that the resulting sympathetic hyperinnervation might increase the propensity for cardiac arrhythmia. The coexistence of denervated and hyperinnervated areas in the diseased myocardium could result in increased electrophysiologic heterogeneity during sympathetic activation, leading to ventricular arrhythmia and SCD.51 Ventricular tachyarrhythmias can be provoked in these patients with heterogeneous remodeling of sympathetic innervation, attributable to nerve growth and degeneration by physical or mental stress or by catecholamine application. On the other hand, adrenergic denervation of viable myocardium may also result in denervation supersensitivity, with exaggerated response of myocardium to sympathetic stimulation and increased vulnerability to ventricular arrhythmias.52,53 Therefore, when assessing the arrhythmogenic potential, variables such as the presence of denervated but viable myocardium, the severity of denervation, the underlying level of sympathetic tone, and the subsequent influences on local ventricular repolarization should be taken into account.
While prevention of arrhythmic deaths is generally ineffective with pharmacologic treatment,54 implantable cardioverter defibrillators (ICDs) reduce the mortality rate in subgroups of patients thought to be at risk.55,56 However, identification of patients who most benefit from these devices remains difficult, and implantation of the device in patients who will not benefit leads to unnecessary morbidity, with increased medical costs.57,58 Current available methods for precise identification of individuals at risk for SCD, such as conventional coronary risk factors, functional impairment, degree of LV dysfunction, and cardiovascular testing analyzing electrocardiographic (ECG) variables and Holter monitoring (frequency of premature ventricular depolarizations, nonsustained and sustained VT) have low accuracy. Additionally, heart rate variability (HRV) and baroreflex sensitivity still have an unknown predictive value. On the other hand, electrophysiologic testing has relatively low sensitivity and positive predictive value.48 Arora et al.59 evaluated the use of 123I-MIBG cardiac imaging (as means of local myocardial sympathetic innervation) and spectral analysis of HRV (as means of central autonomic tone) in patients with ICDs. They studied 17 patients who had ICDs for various indications. The combined use of 123I-MIBG scintigraphy and HRV analysis correlated with the occurrence of an appropriate ICD discharge. Patients with ICD discharges had lower early HMR, more extensive 123I-MIBG defects, and more 123I-MIBG/99mTc-sestamibi (technetium-99m-labeled sestamibi) mismatch segments (denervation in areas of myocardial viability) compared to patients without previous ICD discharge. In addition, the ICD discharge group had reduced values for HRV, suggesting abnormally increased sympathetic tone, when compared with patients without previous ICD discharge. Therefore, the combined noninvasive evaluation of local cardiac autonomic innervation and systemic autonomic function by means of 123I-MIBG and HRV allowed identification of patients at risk for potentially fatal arrhythmias and SCD, who were most likely to benefit from an ICD.
Schäfers et al.60 reported the use of 123I-MIBG SPECT in 25 patients with IVF, demonstrating presynaptic innervation defects in these patients. The same group of investigators subsequently reported the long-term follow-up in 20 of these patients,61 13 of whom showed abnormal 123I-MIBG uptake. During a follow-up period of 7.2 ± 1.5 years, 18 episodes of VF/fast polymorphic ventricular tachycardias occurred in 4 IVF patients with abnormal 123I-MIBG uptake, while only 2 episodes of monomorphic ventricular tachycardia (and no VF) occurred in a single IVF patient with normal 123I-MIBG uptake. Therefore, impaired 123I-MIBG uptake may indicate a higher risk of future recurrent episodes of life-threatening ventricular tachyarrhythmias in patients with IVF.
Simões et al.62 subsequently evaluated 67 consecutive patients within 14 days after acute MI by means of resting myocardial perfusion imaging, 123I-MIBG cardiac imaging, and electrophysiologic parameters. They reported a significant correlation between the extent of sympathetically denervated but viable myocardium and prolonged repolarization, defined by QTc interval in resting ECG, and indexes of delayed depolarization from signal-averaged ECG. However, a significant relation between the presence and frequency of ventricular arrhythmias and the extent of denervated but viable myocardium could not be demonstrated after 4.3 ± 1 years of follow-up. This could be related to the low incidence of ventricular arrhythmias in the study population, and possibly the fact that patients with severely depressed LV function were not included. In a phase 2, open-label, multicenter study that enrolled 50 patients with LV dysfunction and previous myocardial infarction, Bax et al.63 found that late 123I-MIBG SPECT defect score was the only variable which showed a significant difference between patients with and without positive electrophysiological studies. Further studies should demonstrate clinically relevant risk stratification of 123I-MIBG imaging, such as high negative predictive value for life-threatening ventricular arrhythmias, especially in patients with ischemic heart disease and depressed LVEF.
Increase of the electrical heterogeneities of ventricular repolarization contributes to the electrocardiographic phenotype and arrhythmogenicity of ion channelopathies such as the Brugada and long-QT syndromes, despite structurally normal hearts. The ANS plays a prominent role in unmasking these syndromes and precipitating life-threatening ventricular tachyarrhythmias. Both sympathetic and parasympathetic influences on ion channel activity have been found to accentuate electrical heterogeneities, thus contributing to arrhythmogenesis in the long-QT and Brugada syndromes.1
123I-MIBG studies have demonstrated reduced and heterogeneous uptake associated with increased regional WR in patients with long-QT syndrome.64,65 Likewise, Wichter et al.66 reported reduced 123I-MIBG uptake in the inferior and septal LV walls in 47% of patients with Brugada syndrome compared with control subjects, which pointed to presynaptic sympathetic dysfunction of the heart in a large proportion of patients with Brugada syndrome. The pathophysiologic implication of these findings requires further investigation.
Ventricular loss of myocytes and fatty or fibrofatty tissue replacement, resulting in regional or global abnormalities, are the main structural abnormality in arrhythmogenic right ventricular cardiomyopathy (ARVC).67 It has been shown with 123I-MIBG SPECT and 11C-hydroxyephedrine PET that although the LV is not involved in the disease, there is evidence of global and regional denervation in presynaptic catecholamine reuptake and storage of the LV, as well as a reduction in the postsynaptic β-adrenoceptor density assessed by 11C-CGP PET.68–70 Correlation of observed regional abnormalities of 123I-MIBG uptake in the basal posteroseptal regions of the LV with the site of origin of VT (as demonstrated by electrophysiologic testing) has been reported.68,69 These findings suggest a reduced activity of the NE transporter (uptake-1), with subsequent β-adrenoceptor down-regulation, and have potential impact on diagnostic evaluation and therapeutic management of patients with ARVC.
123I-MIBG Imaging in Heart Failure (See Chapter 30)
HF is a complex clinical syndrome characterized by dyspnea, fatigue, and fluid retention, which results from any structural or functional cardiac disorder that impairs the ability of the LV to fill with or eject blood. It is a common, costly, disabling, and potentially fatal disorder. The prevalence and incidence of HF are increasing rapidly in the Western world because of the aging of the population and an ever-increasing number of acute coronary syndrome survivals (CAD is the most prevalent underlying etiology, affecting about 70% of cases), despite advances in different pharmacologic and nonpharmacologic therapies.71
The development of HF initiates with some injury to, or stress on, the myocardium, which usually produces progressive changes in the geometry and structure of the LV with pathologic hypertrophic growth. This pathologic remodeling involves a shift toward glycolytic metabolism, disorganization of the sarcomere, alterations in calcium handling, changes in contractility, loss of myocytes, fibrotic replacement, LV dilation, systolic or diastolic dysfunction, and electrical remodeling (i.e., alterations in the expression or function of ion-transporting proteins, or both) with propensity to malignant ventricular arrhythmia.72 Although several factors can accelerate this process, there is evidence of the important role played by the activation of endogenous neurohormonal systems. Patients with HF have elevated circulating or tissue levels of NE, angiotensin II, aldosterone, endothelin, vasopressin, and cytokines, which can adversely affect the structure and function of the heart.71,72 These neurohormonal factors not only increase the hemodynamic stresses on the LV by causing sodium retention and peripheral vasoconstriction but may also exert direct noxious effects on cardiomyocytes (apoptosis and regression to a fetal phenotype) and changes in the nature of the extracellular matrix (stimulation of myocardial fibrosis), which can further alter the architecture and impair the performance of the failing heart.73,74
The hyperadrenergic state present in HF results in down-regulation and uncoupling of cardiac β-adrenergic receptors, which contributes to progressive impairment of LV systolic function by altering postsynaptic signal transduction. Increased sympathetic tone in HF is directly linked to disease progression, prognosis, and risk of SCD.75 Therefore, noninvasive strategies to determine the state of cardiac autonomic regulation are of significant interest.
The most recent definition of cardiomyopathies makes reference to the presence of HF or electrical disorder. Specifically, they are defined as “a heterogeneous group of diseases of the myocardium associated with mechanical and/or electrical dysfunction that usually (but not invariably) exhibit inappropriate ventricular hypertrophy or dilation and are due to a variety of causes that frequently are genetic. Cardiomyopathies either are confined to the heart or are part of generalized systemic disorders, often leading to cardiovascular death or progressive HF-related disability.”76 Several studies have shown reduced cardiac 123I-MIBG uptake and increased WR in different cardiomyopathies, which could reflect the contribution of the altered cardiac sympathetic nervous function to the development of the myocardial disorder (see Fig. 41-4).
In patients with dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM), Zhao et al.77 found significant correlations of LV function and perfusion with cardiac sympathetic nervous function by means of cardiac 123I-MIBG and 99mTc-tetrofosmin imaging. WR and early uptake of 123I-MIBG resulted in the most significant factors for predicting LV function and LV perfusion, respectively. These investigators indicated that impairment of neuronal uptake function might not be dominant in HCM, since early 123I-MIBG uptake in the nondilated phase of HCM was preserved and correlated significantly with blood flow. On the other hand, in DCM, early 123I-MIBG uptake was decreased, but it was doubtful whether this phenomenon was caused by the low perfusion other than the impaired neuronal uptake function. In patients with DCM, decreased early 123I-MIBG uptake was closely associated with low perfusion. These findings suggest that the kinetics of 123I-MIBG might be different in these two types of cardiomyopathies. It is still unclear whether the decrease of 123I-MIBG uptake is due to sympathetic denervation or to the impairment of neuronal uptake function. 123
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree