Radiation Pneumonitis
INTRODUCTION
The discovery of X-rays by Roentgen in 1895 and of radium by the Curies in 1898 revolutionized medicine at the turn of the 20th century. Roentgen’s first paper on X-rays illustrated the power of diagnostic imaging with a remarkably detailed radiographic image of Frau Roentgen’s hand. As researchers around the world built vacuum tubes and acquired radioactive sources for their studies, it rapidly became apparent that these invisible radiations could produce dangerous, and even lethal, injuries.1–3 Erythema, chronic dermatitis, ulceration, loss of hair, and eye injuries were soon reported in patients who received large doses of radiation during prolonged fluoroscopy procedures. Even greater injuries were reported among the physicians, technicians, and scientists who performed diagnostic procedures or laboratory studies using unshielded X-ray-generating equipment and highly radioactive sources. The development of these radiation injuries suggested that radiation might be useful in the treatment of cancer; indeed, patients with cancer were treated with radiation therapy as early as 1896.1–3
Radiation was found to inhibit the growth of tumors, but this benefit came with the cost of injury to normal tissues within the irradiated areas. Because of the very low energies of the early X-ray and gamma-ray sources, radiotherapy in its early days was limited to using poorly penetrating radiations, which delivered much higher doses of radiation to skin than to even very superficial tumors. As a result, severe early radiation reactions in the skin limited the doses of radiation that could be delivered to tumors. Studies of these skin reactions led to the development of the concept of normal tissue tolerance and an appreciation of the benefits of “fractionated” radiotherapy, using multiple treatments with small doses of radiation.2 The relative sensitivity of the lung to injury from radiation became apparent early in the development of radiation oncology. The clinical syndromes of dyspnea, cough, fever, and radiographic infiltrates occurring weeks to months after irradiation of the thorax were dramatic enough to be described as early as 1922.4
The field of radiation oncology has matured immeasurably over the last century and has incorporated significant advances from fields as diverse as theoretical and applied physics, radiation biology, pathology, cell biology, and immunology.1,2,5–7 The importance of advances in physics and engineering to the maturation of radiation oncology is especially notable.2,7 These advances have led to the development of modern linear accelerators capable of delivering very high-energy, deeply penetrating radiations, which can be used to deliver high radiation doses with great precision to tumors deep within the body. Precise systems for radiation dose measurement, or dosimetry, rapid computers, and precise algorithms for the rapid computerized three-dimensional planning of individualized radiotherapy treatments based on computed tomography (CT) scans and magnetic resonance imaging (MRI) studies have been developed. These advances have changed the dose-limiting toxicities of radiation therapy from painful early reactions in the skin to life-threatening late reactions in the normal tissues invaded by and surrounding the tumors, including the lung.
For clinicians interested in pulmonary medicine, understanding radiation pneumonitis is important. An understanding of radiation injury to the lung can be useful in understanding other lung diseases. Because the chemical mediators of radiation effects, both beneficial and harmful, are free radicals, the pathway leading to radiation injury in the lung overlaps with those leading to many other lung injuries.8,9 In addition, understanding radiation pneumonitis has practical value to physicians in many areas of medicine. Approximately one in three people in the United States will be diagnosed with cancer at some point in their lifetimes. Over half of these patients will be permanently cured of their malignancies. Approximately 65% of all patients with cancer receive radiotherapy at some point in the treatment of their malignancies, and radiotherapy seems destined to remain an important component of cancer treatment for the foreseeable future. Because of this, every physician can expect to care for many patients who are receiving radiotherapy or have received radiotherapy at some point in the past.
In addition to the association of radiation therapy with acute or subacute pulmonary disease, recent studies of plutonium workers have shown an excess incidence of pulmonary fibrosis.10 These findings, which are supported by data from a large number of studies in experimental animals, show that lung injury may be produced by inhalation of insoluble particulate radionuclides that are deposited in lung tissue and produce long-term irradiation of the tissue.
Respiratory diseases are also known to be a cause of increased late morbidity and mortality in the survivors of the atomic bombs in Hiroshima and Nagasaki.11 Therefore, radiation injury to lung is possible in cases in which people are exposed to high levels of inhaled radionuclides or external irradiation through their occupations, accidents, or acts of war or terrorism.9 A working knowledge of the basics of radiobiology and radiation oncology is important to every physician and healthcare provider. An understanding of the potential toxicities of radiotherapy and other exposures to radiation, including radiation pneumonitis, can be critical to patient care.
Many neoplasms involving the thorax are treated with regimens that include the use of radiotherapy to produce either cure or palliation. Radiotherapy is principally a localized, anatomically based modality. The success of radiotherapy hinges on delivering radiation selectively to the sites of malignant disease, while sparing to the maximal extent possible the uninvolved normal tissues.1,2,5 To plan radiotherapy treatments effectively, the radiation oncologist must have a sophisticated appreciation of the malignancy being treated and understand its biologic behavior, patterns of local and metastatic spread, radiosensitivity, and factors that influence the responses of individual patients to therapy. The radiation oncologist must also consider the effects of radiation on normal tissues within the treatment volumes.
Many factors, including radiation dose, fractionation pattern, volume of the tumor and involved margins, prior or planned use of other therapies such as surgery or systemic chemotherapy, and presence of other diseases influence both the probability of controlling the neoplasm and of producing toxic reactions. For cancers of the lung, esophagus, pleura, breast, and chest wall, as well as for lymphomas involving the thorax, optimal treatment frequently involves use of multiple overlapping X-ray beams and possibly electron beams, planned to encompass all of the cancer-containing tissues. Although treatments are carefully planned to include the smallest possible amount of healthy normal tissue, some normal tissue will necessarily be included in the radiation fields. The radiation sensitivity of the specific tissues in the irradiated fields and the acceptable level of risk for complications combine to limit the dose of radiation that can be administered. The planning of radiotherapy always involves a balance of benefit and risk, because the probabilities of controlling the malignancy increase with increasing radiation dose, but the probabilities and severities of the potential complications increase with dose as well.
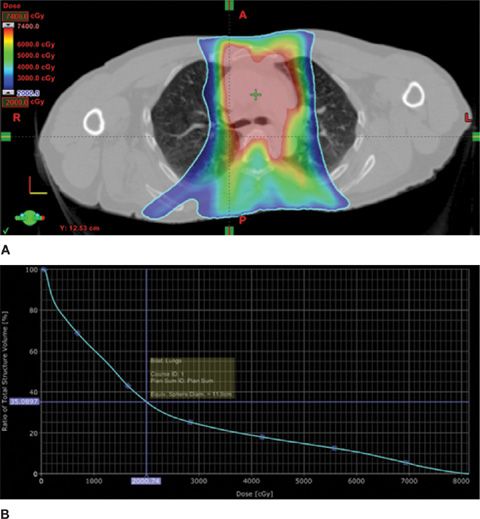
To illustrate the mechanisms involved in planning radiotherapy treatments, a treatment plan is depicted in Figure 59-1A. The first panel shows the isodose distribution for treatment of a stage IIIB non–small-cell lung cancer, using a color-wash display in which the highest radiation dose is shown in red and lower doses in shades of yellow, green, and blue. This represents the sum of multiple radiation portals using intensity-modulated radiation therapy (IMRT), in which computer-based planning is used to deliver high dose to the tumor target with specific dose limits to normal tissue structures, such as the spinal cord, esophagus, heart, and lungs. The volume of normal lung receiving significant radiation can be readily appreciated.
Figure 59-1 A 60-year-old man with stage IIIB non–small-cell lung cancer was treated with radiotherapy and concurrent cisplatin-based chemotherapy. The plans for his radiation treatments are summarized. A. The isodose distribution from a complex multifield radiation plan is overlaid on a treatment-planning CT scan. Using a color-wash format, radiation dose is demonstrated from 20 Gy (green) to 70 Gy (red). B. Cumulative dose–volume histogram of the entire treatment course for bilateral lung tissue. The volume of normal lung receiving 20 Gy or more (V20) is 35%.
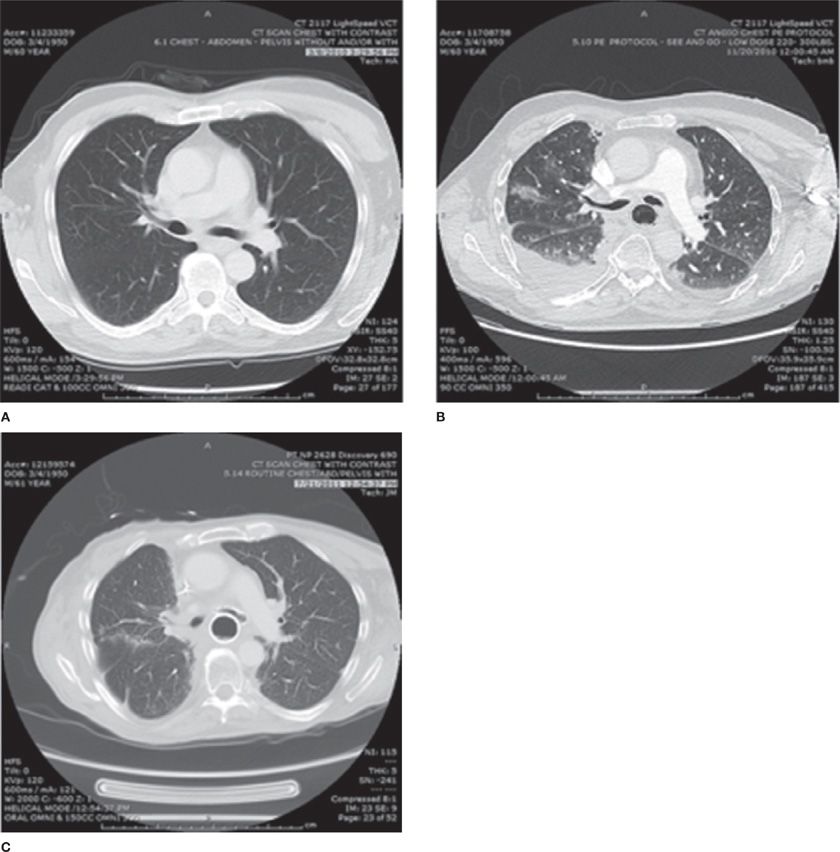
The radiation dose delivered to the lung tissue is shown in a cumulative dose–volume histogram, in Figure 59-1B, which integrates the percentage of the volume of the organ at risk (on the vertical axis) receiving the specified cumulative radiation dose (on the horizontal axis). While this is a simplified representation of a complex dose/volume relationship, this formalism has become important in analyzing radiation dose delivery and correlating dosimetry with treatment outcome. In the illustration, the volume of lung receiving greater than or equal to 20 Gy is referred to as the V20. In this case, the V20 is 35%, which predicts at least a 25% risk of grade 2 or greater pneumonitis (see discussion below, Clinical Syndromes). In Figure 59-2, the patient’s pretreatment CT scan (panel A) is shown in comparison to a 3-month postradiation scan (panel B). The latter demonstrates radiation-induced inflammatory changes corresponding to the high-dose region of the radiotherapy. In this case, these changes were associated with increasing dyspnea, cough, and a decrease in diffusion capacity. Panel C shows slowly resolving changes, with persistent pulmonary fibrotic changes, on a CT scan obtained 1 year post treatment.
Figure 59-2 The same patient as in Figure 59-1, developed a progressive dyspnea and nonproductive cough. A comparison of chest CT imaging from before treatment (Panel A) and 3 months after treatment (Panel B) demonstrated interstitial infiltrates and ground-glass changes in a distribution consistent with the prior radiation, as well as the development of new pleural effusions. This is consistent with radiation pneumonitis, and the patient was treated with corticosteroids. He symptomatically improved over several months. A chest CT scan at 1 year (Panel C) shows residual fibrotic changes in the paramediastinal region. Panels (B) and (C) also show bronchial and esophageal stents placed for palliation of a tracheoesophageal fistula.
In the case presented, if the malignancy is cured, or the patient experiences the desired improvement in symptoms with minimal or manageable toxicity from the radiotherapy, the treatment is a success even if accompanied by radiographic changes or by other subclinical damage to the lung or other organs. Overt pulmonary toxicity is, however, a potential consequence of thoracic radiotherapy that sometimes overshadows the benefits of treatment.
BRIEF OVERVIEW OF RADIOLOGIC PHYSICS
External beam radiotherapy is generally delivered using X-rays or gamma rays. Both of these radiations are high-energy electromagnetic waves or photons that are able to cause ionizations when interacting with matter.7 The only difference between them lies in the manner in which they are produced: Gamma-ray photons are emitted from atomic nuclei during the decay of radioactive atoms, and X-rays are produced when high-energy electrons strike a target material and interact with the electron shells of atoms in that target, causing them to emit X-ray photons (the bremsstrahlung effect). After its emission, an individual X-ray photon is indistinguishable from a gamma-ray photon. Thus, although the discussion below uses X-rays as examples, the principles are equally applicable to radiotherapy using high-energy gamma rays (e.g., from cobalt-60 teletherapy units or brachytherapy using implanted radioactive sources).
The X-rays used for diagnostic imaging are in a relatively low-energy range, in which the dominant interaction of photons with matter is through the “photoelectric effect.” In this process, absorption of a photon causes an electron to be ejected from the inner shell of an atom. The probability of photoelectric interactions increases as a function of the cube of the atomic number, that is, as Z.3 Consequently, large, heavy atoms absorb low-energy diagnostic X-rays much more efficiently than smaller, lighter atoms.
Diagnostic radiology capitalizes on the large differences between the absorption of low-energy X-rays in materials with different compositions, for example, air, soft tissue (which is 70% water and, therefore, composed primarily of the small atoms hydrogen and oxygen), bone (with its high calcium content), and administered contrast agents containing barium, iodine, or other heavy atoms. The differences in absorption are used to image anatomical structures. In contrast, high-energy X-rays used in radiotherapy interact with matter primarily by a phenomenon called the “Compton effect,” in which X-rays cause ionization of atoms via interactions with their outer electron shells. The Compton effect is not dependent on the atomic number, but is, instead, a function of the electron density. Because the electron densities of most biologic tissues are relatively uniform, for the purposes of most radiotherapy dosimetry, it is reasonable to assume that a patient is of uniform density, equivalent to water.
An important caveat to radiation dosimetry involves the standard specification of doses in tissues that include a large proportion of air, such as the lung. As a single X-ray beam penetrates through water or tissue, the dose received by the tissue falls progressively, generally as an exponential function of distance. Because of its markedly lower density, air absorbs less radiation energy and, therefore, attenuates the X-rays less than does tissue or water. With the quantitative knowledge of lung density that can now be derived from CT scanning, algorithms have been devised to estimate the inhomogeneity in the absorbed dose resulting from differences in the density of lung and other soft tissues.7,12 These heterogeneity corrections show that routine dosimetric calculations, which assume uniform density, underestimate the radiation doses to lung and tissues beyond the lung by 5% to 25%.
Although the effect of tissue heterogeneity is a very important consideration when quantifying the radiation dose delivered to the lungs, one must remember that doses delivered to the thorax and the lungs historically have been reported in the medical literature without heterogeneity corrections. Moreover, because the preponderance of clinical data concerning lung tolerance have been determined and reported using older dosimetric algorithms, which assume that lung has water-equivalent density, the impetus to change dose reporting is tempered by the desire to avoid confusion between the newer and older literature. The reader should assume, unless explicitly stated otherwise, that the historic radiation doses given in this chapter, or for that matter any publication, are not necessarily corrected for lung density. Most modern radiotherapy planning systems now include the ability to account for tissue heterogeneity. Without such corrections, actual dose delivery to the chest region is modestly higher than the nominal doses reported. However, the variability of the actual dose delivery is highly individual and only recently has been accounted on a routine basis with improvements in computerized treatment planning.
Radiation dose is currently reported using the unit of the Système International (SI), the gray (Gy). The Gy is a measure of the energy absorbed by 1 kg of tissue; 1 Gy = 1 J/kg. The former unit of absorbed dose, called the “rad” (an acronym for “radiation absorbed dose”) was measured with the CGS system; by definition, 1 rad = 100 ergs per gram. To compare old and recent literature, one must remember that 1 Gy = 100 rad. Despite the fact that it is not an approved SI unit, some radiotherapy literature avoids this conversion by giving the dose in centigray (cGy), where 1 cGy = 0.01 and Gy = 1 rad. Other measures of radiation dose seen in the literature include the roentgen, the Sievert, and the rem.
The roentgen measures radiation exposure, rather than energy absorption, and refers specifically to the amount of ionization produced in air under standard conditions (1 R = 1 electrostatic unit/cc = 2.58 × 10–4 coulombs/kg of “standard air” at a density of 1.29 × 10–4 g/cm3 at 0°C and 760 torr). This unit is frequently encountered in the radiation dosimetry literature, not only because it was historically used as a measure of dose, but also because many widely used radiation monitors (e.g., ionization chambers) directly measure radiation exposure at the surface of the body. The dose absorbed by tissue is then calculated from this exposure.
The radiation protection literature uses the unit of “equivalent dose,” the Sievert (Sv), which is calculated as the absorbed dose (in Gy) multiplied by a “weighting factor” that considers the differing biologic effects of different radiations. Although the weighting factors for some radiations, such as neutrons and alpha particles, can be as high as 20, the weighting factors for X-rays, gamma rays, and electrons are all defined as 1. For most purposes in diagnostic and therapeutic radiology, therefore, 1 Sv = 1 Gy. The Sv replaces the older unit of equivalent dose, the rem (1 Sv = 100 rem). Unfortunately, the literature on radiation-induced lung injury includes papers using all of these different units, creating great confusion for readers. For simplicity, all doses in this chapter have been converted to Gy.
RADIOBIOLOGY OF RADIOTHERAPY
When X-rays pass through tissue, a complex series of physical and chemical reactions occurs.1,13,14 As the X-rays interact with atoms along their path, energy is absorbed, and energetic fast electrons are ejected. These fast electrons travel through tissue, producing secondary ionizations, which lead within milliseconds to the generation of a variety of highly reactive free radical species. Because biologic materials are about 70% water, ions and free radicals derived from water (e.g., H, OH, H2O+, H3O+) are the main reactive species produced. These ions and radicals react with each other and with other nearby molecules, creating a wide variety of chemically reactive species and producing many kinds of damage in biologic macromolecules. Because DNA contains information that is critical to the cell, while most other molecules can be replaced readily, damage to DNA is the most important biologic effect of irradiation. Radiation produces a wide variety of lesions in DNA, including single- and double-strand breaks, damaged bases and loss of bases, as well as chromosomal breaks and rearrangements. If these lesions are not repaired, the result can be permanent mutations or changes in chromosomal structure that lead to the death of the cell or changes in its behavior.
The cytotoxic effects of radiation are the basis for both the antineoplastic effects and the toxicities of radiotherapy. A theoretical concern is that radiotherapy may produce a mutation in a previously normal cell that leads to the development of a new malignancy. Although radiation-induced malignancies do occur and are a primary concern in considerations of environmental and occupational exposures,1,11,13 malignant transformation is, fortunately, a rare enough event at the doses used in radiotherapy that the risk of inducing a second cancer in an individual patient is very small relative to the great benefit of curing the existing malignancy.5 The greater risk to the patient lies in the fact that radiation is not selectively toxic to the tumor cells, but instead, kills both normal and malignant cells within the treatment field.
Although the radiochemical reactions that lead to cytotoxic damage are complete within milliseconds after the end of irradiation, cells dying from radiation injury do not die immediately. In fact, soon after irradiation, radiation-sterilized cells are indistinguishable from cells that ultimately survive irradiation in their appearance, metabolic activities, and even rates and patterns of proliferation. Most radiation-sterilized cells ultimately die during mitosis, but they may first undergo one or even several divisions, producing an abortive clone of sterile cells, all of which ultimately die and disintegrate through apoptosis, necrosis, mitotic catastrophe, senescence, autophagy, or other pathways of cell death.15,16 This delayed cytotoxicity underlies many of the effects seen in radiotherapy. Rapidly growing tumors, for example, generally begin shrinking sooner than slowly growing tumors, and many tumors continue to shrink progressively for months after radiotherapy.13,17 Analogously, radiation reactions in normal tissues reflect the normal patterns of cell turnover in the tissue.
After irradiation, nonproliferating, terminally differentiated cells continue to perform their differentiated functions throughout their normal life spans. Other cells that are not proliferating at the time of irradiation likewise continue to function normally until they are recruited into proliferation, perhaps months or even years after irradiation; when they begin to proliferate, their progeny die. Rapidly proliferating cells, such as mucosal or intestinal epithelium or nucleated blood and bone marrow cells, die within a few days of irradiation, leading to the familiar early radiation reactions of epilation, desquamation, mucositis, and hematologic depression.3,5 Some cell types, especially hematopoietic cells, may be induced by radiation-induced damage to enter a pathway of programmed cell death that leads to apoptosis; the role of early and delayed apoptosis in determining the response of tumors and normal tissues to radiotherapy is the subject of intensive investigation.
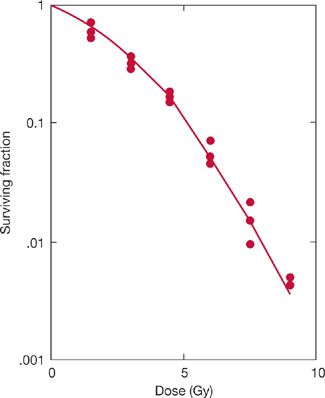
A typical survival curve for mammalian cells, obtained using mouse lung cells, is shown in Figure 59-3. As a first approximation, cell survival falls exponentially as the radiation dose increases. Statistically, this implies that each incremental dose of radiation has the same cytotoxic effect; that is, each incremental dose kills the same proportion of the viable cells that were present in the population at the beginning of that irradiation. Very low doses of radiation have somewhat lesser effects; the shoulder on the cell survival curve reflects the ability of the cells to accumulate and tolerate or repair some of the damage produced by radiation.
Figure 59-3 Survival of lung cells treated with different doses of radiation. Cells were explanted from mouse lungs, irradiated in vitro, and assayed for viability using a colony formation assay. (Reproduced with permission from Guichard M, Deschavanne PJ, Malaise EP. Radiosensitivity of mouse lung cells measured using an in vitro colony method. Int J Radiat Oncol Biol Phys. 1980;6(4):441–447.)
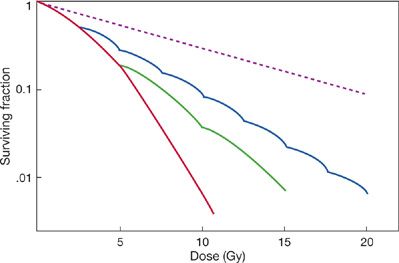
The effect of the repair of radiation damage can be seen when the radiation dose is divided into two or more treatments separated by hours or days, rather than being delivered in a large single dose. Dividing, or “fractionating,” the radiation dose allows cells to repair damage to their DNA and to proliferate between treatments.1,5 As a result, there is less cytotoxicity from a fractionated treatment regimen than from the same total radiation dose delivered as a large single fraction (Fig. 59-4). Smaller fractions produce less cytotoxicity than larger fractions. Similarly, the cytotoxic effects of radiation are diminished when the radiation is delivered continuously at a low-dose rate, over hours or days, allowing repair and proliferation to occur during irradiation (Fig. 59-4).
Figure 59-4 Effect of fractionated irradiation and low-dose rate irradiation on cell survival. The survival curve for lung cells treated with a single dose of radiation is redrawn from Fig. 59-3. The calculated effect of dividing the radiation dose into several daily treatments with 5 Gy/fraction or 2.5 Gy/fraction is illustrated. The dashed line illustrates the survival curve that would be expected for irradiation delivered continuously at a low-dose rate over several hours, allowing repair and proliferation to occur during treatment. Changes in the cytotoxicity of radiation with fractionation and at low-dose rates lead to decreased injury in lungs irradiated with analogous regimens.
Fractionating therapeutic irradiations or delivering the radiation at low-dose rates generally appears to increase the therapeutic ratio by protecting normal tissues against radiation injury, while producing a smaller increase in the relative radioresistance of the tumor; treatment outcomes are thereby improved. This increase in the therapeutic ratio is thought to reflect qualitative and quantitative differences between normal and malignant cell populations, including differences in the intrinsic radiosensitivity of the critical cells and in the patterns of cell proliferation and cell loss, as well as differences in the ability of the normal and malignant cells to repair radiation damage. Empiric observations of patients treated with radiotherapy, laboratory experiments with tumors and normal tissues in rodents, and studies with cells in culture have all been used to guide the development of the clinical fractionation schedules now in use. This optimization process is ongoing and will undoubtedly continue, incorporating new information about the repair of radiation damage in normal and malignant cells and about the physiologic factors that modulate development of late radiation injuries in specific normal tissues. In addition, efforts will continue in the development of new technologies for targeting and delivering radiation.
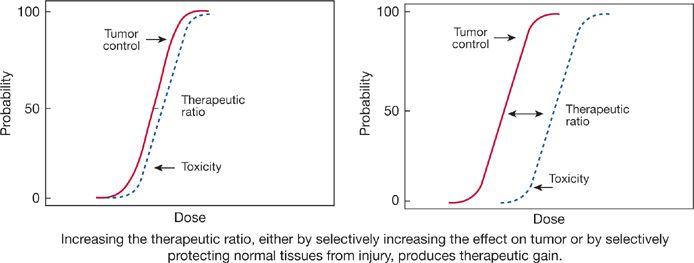
In this process, as in any change in cancer therapy, the critical parameter is the therapeutic ratio (Fig. 59-5). A new treatment regimen is superior only when it produces an increased effect on the tumor without an equivalent increase in toxicity to critical normal tissues, reflected in an increase in the therapeutic ratio and therapeutic gain. The art of radiotherapy lies in the design of treatment fields that minimize radiation doses to normal tissues and in the development of treatment regimens that use all available information on the biology of the tumor and of the critical normal tissues.
Figure 59-5 The therapeutic ratio is the critical factor determining the success of cancer therapy.
PATHOPHYSIOLOGY OF RADIATION PNEUMONITIS
Much of our current understanding of the pathophysiology of radiation injury to the lungs is derived from animal experimentation. Translation of animal data to human conditions is always problematic, because differences in the biology and physiology of different species may preclude direct and definitive extrapolation from animals to humans.9,13,18 Instead, studies with experimental animals must be designed to identify physiologic factors and biologic mechanisms that can be used to interpret clinical data and suggest avenues for clinical investigations.
Data on radiation pneumopathy in humans is fragmentary and complicated by the variability in the patients treated with thoracic irradiation. Most studies of radiation pneumonitis include patients with a variety of malignancies who have been treated with different irradiation regimens, often in combination with chemotherapy and surgery. Moreover, patients vary widely in age and the presence of other diseases and risk factors. Therefore, our current understanding of radiation injury to the lung remains incomplete. What is known suggests a complex, multifactorial mechanism of injury and disease progression that reflects cytotoxic effects on both epithelial and endothelial tissues. Inflammatory responses involved in this injury cascade include transient increases in reactive oxygen and nitrogen species, macrophage infiltration and activation, oxidative stress, induction of interstitial fibrosis with regional tissue hypoxia, and disordered cytokine and cellular signaling—including profibrogenic transforming growth factor-beta (TGF-β), proangiogenic hypoxia-inducible factor-1 alpha (HIF-1α), and vascular endothelial growth factor (VEGF).8,9,14,19–25 Similarities to lung injuries resulting from cancer chemotherapy, other drugs, inhaled chemicals, oxygen toxicity, immune reactions, and idiopathic pulmonary fibrosis are intriguing, especially when one considers that many of these diseases include pathologic responses to free radical chemical species and are likely to reflect similar underlying initial lesions.
Partial-lung resection and localized irradiation have certain similarities: Their effects are largely localized to the treated areas and, consequently, depend on the number of pulmonary lobules or alveolar–capillary units functionally destroyed. Thus, the volume of lung irradiated is an important determinant of toxicity. Consequently, the radiation oncologist plans treatment to minimize the volume of lung receiving high radiation doses, just as the thoracic surgeon plans lung resection with due consideration to anticipated residual lung function. Of course, this simple analogy has its limitations. For example, inactivation of enough lobules by radiation increases the ventilatory dead space and could lead to shunting and ventilation–perfusion mismatching. However, in clinical practice, extensive shunting generally is not observed.26 In fact, postradiation radionuclide ventilation–perfusion scans tend to show both underperfusion and underventilation that is dose dependent in irradiated areas of partially irradiated lungs.27 In most cases, radiation injury in lung conforms to the radiation treatment fields, but in some, effects outside the treated areas are observed, with localized radiation inducing a more generalized or diffuse hypersensitivity pneumonitis.28
The effects of radiotherapy on the lung reflect the proliferation patterns of the different cellular components of the terminal capillary–alveolar units.29 Type I pneumocytes are the dominant epithelial cells of the lung, covering about 83% of the alveolar surface. Type I pneumocytes are normally nonproliferating and do not proliferate in response to injury. Consequently, they are thought to be relatively resistant to the cytotoxic effects of radiation.
Type II pneumocytes, which comprise about 16% of the cells in the human lung, are the principal source of surfactant that modifies alveolar surface tension to prevent atelectasis. Type II pneumocytes have turnover times of about 1 month. In response to certain injuries, these granular pneumocytes can be induced both to undergo rapid mitosis and differentiate into type I pneumocytes.
Endothelial cells comprise about 30% of the cells in human lungs and form a continuous layer between the blood and the lung tissue. Although endothelial cells are classified in most tissues as stromal cells, endothelial cells in lung are actually parenchyma, because they are critical to the function of this organ. Capillary endothelial cells are a constantly renewing population, with an estimated turnover time on the order of 2 months. Endothelial cells can be induced into rapid compensatory proliferation after injury; therefore, radiation may result in depletion of both type II pneumocytes and endothelial cells.
Several lines of evidence suggest that radiation injury is related primarily to cytotoxic damage, especially to the surfactant-producing type II pneumocytes and vascular endothelial cells. Although clinical signs of pneumonitis require weeks to develop, laboratory studies reveal evidence of lung injury within hours after large single doses of radiation.6,22,30–32 Shortly after irradiation, electron microscopy may detect abnormalities in surfactant-containing lamellar bodies. There is an increase in surfactant in bronchoalveolar lavage specimens within hours of irradiation that persists for several weeks. Ultrastructural evidence of endothelial cell damage is also seen soon after lung irradiation, and a rapid increase in capillary permeability occurs, reflecting loss of integrity of cell junctions, intracellular vacuolization, cellular pleomorphism, and sloughing of the basement membrane. Capillary occlusion by cellular debris and microthrombi may occur at high doses.
The clinical course of lung injury occurs later and includes a pneumonitic phase, developing weeks to months after radiation, followed by a fibrotic phase, developing months to years later. To explain the two clinical phases, Rubin and Casarett’s original model of radiation lung toxicity suggested that the pneumocytes and endothelium represented two separate and distinct cellular targets, and that damage to pneumocytes led to pneumonitis, while vascular damage led to fibrosis. This older model is now thought to be incorrect; current data19,20,22,29,33–38 suggest that the pneumonitic and fibrotic processes both are manifestations of a common pathway of injury and response.
Histologically, one can recognize a typical sequence of events developing in the lung after large doses of radiation.6,21,30 Within days to weeks, vascular congestion and intra-alveolar edema and exudation occur, followed by infiltration of inflammatory cells and epithelial desquamation. Weeks later, collagen fibrils are deposited within areas of injury and interstitial edema, leading to a thickening of alveolar septa similar to that in hyaline membrane disease. The probability and severity of these changes are quite variable and depend on such factors as the radiation dose and treatment volume. The severity of the damage and volume of tissue affected determine whether a pneumonitic picture becomes clinically evident. Resolution of inflammatory infiltrates and alveolar exudates, which can be improved by anti-inflammatory agents such as glucocorticoids, correlates with symptomatic improvement and resolution of radiographic opacities in the affected lung.
Inflammatory cells, particularly alveolar macrophages, migrate into areas of radiation injury. This induces an ensuing cytokine cascade and mediates the host response,9,20,22,25 similar to that which occurs in other inflammatory conditions, which can lead to pulmonary fibrosis.
Rubin et al. have detected a biphasic increase in mRNA expression for the proinflammatory cytokines IL-1α, IL-1β, and TNF-α at 2 and 8 weeks after radiation.22 Preliminary clinical trials also suggest that elevated serum levels of IL-6 before and during radiotherapy predict an elevated risk of radiation pneumonitis. Beginning 24 hours following radiation, TGF-β, a cytokine that mediates fibrotic responses, increases.21 Elevated levels of VEGF may be detected 2 weeks later. Tissue hypoxia accompanies the inflammatory response. Clinical data implicating TGF-β as a predictive marker for pneumonitis, however, have been mixed. Collagen gene expression is also appreciably increased corresponding to the fibrotic changes seen histologically. These studies suggest that early and persistent elevations of cytokine production and alterations of intercellular signaling are critical to the development of radiation reactions in the lung. There is increasing evidence from studies with inbred mice that genetic differences, age, and past health and treatment history modulate the development and severity of fibrosis and hyaline membrane formation, thus determining the nature of the late toxic lesion and the time of development of radiation pneumotoxicity.9,33,34
The processes described earlier lead to pathologic changes that conform spatially to the areas to which localized radiation was administered. Interestingly, it has been discovered that radiation can also induce an allergic alveolitis. This is observed infrequently as a diffuse pneumonitis, or occasionally as a patchy, transient bronchiolitis obliterans organizing pneumonia (BOOP; see Chapter 57) occurring outside the treated fields. In its most severe form, the result is the acute respiratory distress syndrome (ARDS; see Chapter 141). Morgan and Breit28 have suggested that this form of radiation-induced pneumonitis be termed “sporadic”. The occurrence of the syndrome actually may be more common than appreciated. One series showed a 2.3% incidence of BOOP in women undergoing whole breast radiotherapy, occurring outside the radiotherapy fields four or more months after exposure.39 Bronchoalveolar lavage in humans and in experimental animals frequently shows a significant increase in activated T-helper (CD4+) lymphocytes, temporally related to irradiation and occurring equally in the irradiated lung and the contralateral, nonirradiated lung. Gallium scanning may also show bilateral uptake not corresponding to the treated regions. Frequent reports of autoantibodies, including antibodies to collagen, in the sera of patients with cancer even before treatment suggest the possibility that malignancy-associated autoimmune reactions may be involved in the syndrome.
CONFOUNDING EFFECTS OF CHEMOTHERAPY
Many cytotoxic drugs employed as antineoplastic agents can produce pulmonary toxicity (see Chapter 65).40–42 Bleomycin, which has been extensively studied, kills cells by generating reactive free radical species similar to radiotherapy and may give rise to both pneumonitis and fibrosis.42 Doxorubicin, mitomycin C, irinotecan, and gefitinib have been associated with lung toxicity, as have the antimetabolites (methotrexate, cytosine arabinoside, gemcitabine, fludarabine, and the nitrosamines) and the podophyllotoxins (etoposides, paclitaxel, and docetaxel). Interestingly, lung injury has also been reported after treatment with immune modulators, including interferons, IL-2, and TNF-α.
As high-dose alkylating agent chemotherapy is used more frequently in the setting of bone marrow or peripheral stem cell transplantation, agents such as cyclophosphamide, BCNU, and busulfan have been associated increasingly with clinically significant pneumonitis. The direct toxicity of many widely used anticancer drugs to the lungs sounds a note of caution for those considering development of treatment protocols combining systemic chemotherapy with lung irradiation. Moreover, the concurrent administration of antineoplastic agents and radiotherapy may make it difficult to discern to what degree pulmonary injury in an individual patient is related to radiotherapy alone.
Animal studies addressing changes in respiratory rates and/or death resulting from lung injury show that the severity of the lung injury can be increased when doxorubicin, bleomycin, cyclophosphamide, mitomycin C, dactinomycin, or vincristine are administered along with radiation.43,44 No enhancement has been documented in studies with 5-fluorouracil, cisplatinum, carboplatinum, hydroxyurea, vinblastine, or methotrexate, despite reports of lung toxicity from methotrexate alone.
As a wide variety of cytokines and molecularly targeted agents are now available for pharmacologic administration, modulation of radiation injury by these biologic agents needs increased study. Interferons have been shown both to increase and decrease radiation lung toxicity, whereas interleukins 1 and 2 may have protective effects. Some radiation-drug interactions in the lung have been shown to be schedule dependent, with the effect of the combination varying with the sequence and the time between treatments with the two agents.43,44 Additive, subadditive, and even supra-additive toxicities may be observed in rodents when single treatments with the same dose of radiation and drug are given over a 24-hour period, but in different sequences and different times between treatments. Such findings highlight the complexities of combined-modality therapy and the difficulty of using animal data to plan clinical treatment regimens.
Data from several specific clinical situations show that regimens combining radiation with particular chemotherapy agents can produce significant risks of pneumonitis. As summarized in reviews of chemotherapy and radiation-induced pneumonitis, docetaxel, mitomycin C, gemcitabine, and irinotecan given concurrently with radiotherapy seem to elevate the risk of pneumonitis or lung toxicity.45,46 On the other hand, drugs that are commonly used in lung cancer concurrent with radiotherapy, such as cisplatin, carboplatin, paclitaxel, and etoposide, do not consistently elevate the risk of pneumonitis; alternatively, the clinical data regarding pneumonitis risk so commonly include these chemotherapy agents that the risk may already be incorporated into consideration.47–51 Older clinical data from pediatric trials strongly suggest that administration of concurrent doxorubicin or actinomycin D with thoracic radiotherapy generally should be avoided or, alternatively, that the radiation doses should be reduced significantly where these drugs are used.
Sequential treatment with doxorubicin or actinomycin D and radiation is less likely to produce lung injury. However, a phenomenon termed “radiation recall” has been well described, in which either of these two drugs given even several months after radiotherapy will produce an inflammatory reaction in the region corresponding to the radiation treatment fields.52 Although this reaction is best known in skin, it also has been well documented in the lungs in several case reports and has been produced in experimental animals. Radiation recall probably reflects the fact that the irradiated areas of the lung still retain residual, subclinical injury, which is exacerbated into clinical pneumonitis as a result of the additional injury from the drug. Therefore, the biologic basis of the recall phenomenon is analogous to that of the residual radiation injury, which decreases the ability of heavily irradiated lung tissue to tolerate a second course of radiotherapy delivered months or years later.5,6,37
CLINICAL SYNDROMES
Radiation oncologists conventionally divide clinical toxicities into acute and late effects,5,6 with both radiation pneumonitis and fibrosis considered late toxicities. Several grading systems for pneumonitis have been developed for scoring lung injury during clinical trials (Table 59-1).