The influence of race on quality of anticoagulation control is not well described. We examined the association between race, international normalized ratio (INR) monitoring intensity, and INR control in warfarin-treated patients with atrial fibrillation (AF). Using data from the Veterans Health Administration (VHA), we performed a retrospective cohort study of 184,161 patients with a new diagnosis of AF/flutter from 2004 to 2012 who received any VHA prescription within 90 days of diagnosis. The primary predictor was race, ascertained from multiple VHA and linked Medicare demographic files. The primary outcome was first-year and long-term time in therapeutic range (TTR) of INR 2.0 to 3.0. Secondary outcomes were INR monitoring intensity and warfarin persistence. Of the 116,021 patients who received warfarin in the cohort, INR monitoring intensity was similar across racial groups. However, TTR was lowest in blacks and highest in whites (first year 0.49 ± 0.23 vs 0.57 ± 0.21, p <0.001; long term 0.52 ± 0.20 vs 0.59 ± 0.18, p <0.001); 64% of whites and 49% of blacks had long-term TTR >55% (p <0.001). After adjusting for site and patient-level covariates, black race was associated with lower first-year and long-term TTRs (4.2% and 4.1% below the conditional mean, relative to whites; p <0.0001 for both). One-year warfarin persistence was slightly lower in blacks compared to whites (58% vs 60%, p <0.0001). In conclusion, in patients with AF anticoagulated with warfarin, differences in INR control are most evident among blacks, underscoring the need to determine if other types of intensive management or warfarin alternatives may be necessary to improve anticoagulation among vulnerable AF populations.
In the United States, more than 2 million adults have atrial fibrillation (AF), and the prevalence is anticipated to increase to 6 million by 2050. Stroke and death are the most significant complications of atrial fibrillation, and therapeutic international normalized ratio (INR) levels from warfarin anticoagulation reduce the relative risk of stroke by approximately 60%. Therefore, time in therapeutic range (TTR) has been widely adopted as a process and quality measure by multiple stakeholders (National Quality Foundation). Nonetheless, variation in INR control between clinical centers and between countries has been observed in epidemiologic studies and clinical trials. Although this could indicate site-level variation in care structures, given geographic variation in race it could also indicate racial differences in TTR. As such, questions regarding the influence of race on quality of anticoagulation control remain unanswered. We examined the association between race and TTR in a large national cohort with AF. We also assessed whether INR monitoring intensity and warfarin persistence vary by race.
Methods
The Retrospective Evaluation and Assessment of Therapies in AF cohort is a retrospective cohort of patients with newly diagnosed AF from the Veterans Administration (VHA) health care system. The VHA is the largest integrated health system in the United States. We used the following centralized, comprehensive VHA data sets of care: (1) the VHA National Patient Care Database, (2) the VHA Managerial Cost Accounting national pharmacy data set, (3) the VHA Fee Basis inpatient and outpatient data sets, (4) Medicare inpatient and outpatient institutional claims data (part A, part B, and carrier files), and (5) the VHA Vital Status File. The study was approved by the local institutional review board.
Methods regarding cohort construction have been previously described in detail. Our cohort included patients with newly diagnosed AF ( International Classification of Diseases, Ninth Revision 427) associated with an inpatient or outpatient VHA encounter (“index AF diagnosis”). The initial Retrospective Evaluation and Assessment of Therapies in AF cohort included patients with newly diagnosed AF from October 1, 2003 to September 30, 2008 (VHA fiscal years 2004 to 2008) but was subsequently expanded to also include VHA fiscal years 2009 to 2012. Patients were required to have a second confirmatory AF diagnosis within 30 and 365 days of the index AF diagnosis. Patients had to have at least one primary care, cardiology, women’s health, nephrology, geriatric, or anticoagulation clinic VHA outpatient visit in the continental US and receipt of a VHA outpatient prescription within 90 days of the index AF diagnosis.
To account for Medicare-eligible veterans who could be receiving part of their anticoagulation care outside of the VHA, we used a previously validated method to link patients with their Medicare inpatient and outpatient claims data for the corresponding time period.
The primary predictor was race (white, black, Asian, native Hawaiian/Pacific Islander, American-Indian/Alaska Native, or multiracial). VHA medical record race information was augmented with self-reported race data from the VHA Vital Status Master File and the Centers for Medicare and Medicaid Services. We used a previously validated method of calculating race, in which race was calculated multiple times, each time relying on different race data to verify high agreement regardless of whether the source data were self-reported, proxy-reported, or observer-reported, as well as whether the source year differed. If multiple races were coded across different claims, we then tabulated all race values and assigned the most frequent value. In the event of a tie, we assigned the most recent value. This “most frequent/most recent” algorithm has also been previously validated at the VHA. We retained patients with missing race data to evaluate for informative missingness. Of the 184,161 patients in our study, only 0.8% had race unclassified.
The primary dependent variables were first-year and long-term TTR. Secondary dependent variables were (1) first-year and long-term INR monitoring rate (INRMR) and (2) one-year warfarin persistence, defined as the proportion of patients on warfarin at 1 year from their index AF diagnosis. Our approach has been validated and used previously with VHA data.
We calculated the TTR using a modification of the Rosendaal method. We used INR values from outpatient and inpatient VHA files. We excluded periods of VHA and Medicare inpatient hospitalizations from the days eligible for TTR analyses because warfarin is frequently not administered during hospitalizations and because inpatient warfarin management may not reflect outpatient care processes or quality. However, we included inpatient INR values on the date of inpatient admission if the patient was on warfarin within the previous 30 days leading up to the hospitalization to increase the sensitivity of identifying supratherapeutic or subtherapeutic INR values because such out-of-range values could have precipitated the admission.
Two TTR values were calculated for each patient: (1) for the first 12 months after the index AF diagnosis (first year) and (2) from the index AF diagnosis until warfarin discontinuation or administrative censoring (long term). We calculated these separately because INR lability is often greater in the first year after warfarin prescription compared to long term or steady state care.
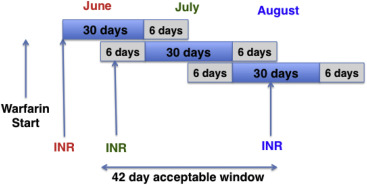
Outpatient and inpatient INR VHA laboratory data, as well as VHA outpatient encounters and events data and outpatient fee basis data, were used to calculate first-year and long-term INRMRs. Guidelines recommend that patients have INR monitoring once every 4 weeks, although there may be more frequent monitoring after initiation of warfarin. As in previous reports, we extended the acceptable period of monitoring to a 42-day interval, consisting of a 30-day block with 6 additional days on the beginning and end of the block ( Figure 1 ). The rationale is to allow for situations in which adequately monitored patients do not have INR monitoring strictly by calendar month. INR monitoring at a minimum of every 42 days has been recommended as a quality measure for the minimum acceptable monitoring frequency, including in the VHA health care system.
Although outpatient INR tests provided most data for INR monitoring episodes, we also used inpatient INR tests that were performed on the date of discharge. To avoid potential underestimation of INR monitoring intensity, we also included INR testing claims identified from VHA Fee Basis outpatient claims. We did not count INRs performed on the day of admission because they typically would be performed as part of admission laboratories (e.g., in the emergency department) rather than as part of routine monitoring. We restricted our measurement of INRMR to include only INRs drawn during periods of warfarin exposure. INR eligibility was determined by evaluating outpatient warfarin dispensation from the VHA Decision Support System (DSS) files. Active treatment with warfarin was determined by filling of warfarin prescriptions. The period of active use is determined on the basis of the prescribed days of supply. A grace period of 30 days between prescriptions was allowed to prevent underestimation of the length of active treatment. Inpatient days were excluded from analysis. Because our cohort was comprised patients newly diagnosed with AF, we excluded patients who had been on warfarin for <90 days for a given fiscal year to obtain a clinically meaningful INRMR.
Baseline co-morbidities were identified up to 2 years before the date of first AF diagnosis using National Patient Care Database, fee basis outpatient care, and inpatient encounter files. We also calculated measurements of stroke risk (CHADS2 score), warfarin bleeding risk (Anticoagulation and Risk Factors in Atrial Fibrillation [ATRIA] score), and co-morbidity burden (Selim and Charlson indexes) as previously described.
Because distance to care could affect likelihood of warfarin prescription and INR monitoring, we calculated the distance (in miles) from the center of the patient’s home zip code to the nearest VHA medical center (to approximate distance to cardiology care) and to the nearest VHA outpatient care facility (to approximate distance to primary care). We also examined the difference between these 2 distances as we have previously shown that this explains variation in the use of primary care versus cardiology specialty care for newly diagnosed AF.
We compared differences in baseline characteristics between patients of different races using chi-square tests for categorical variables and t tests for continuous variables. For the primary outcome of TTR, we kept the outcome variable as continuous because the relation between TTR and stroke or hemorrhage would be expected to be continuous. Additionally, although 60% TTR has been demonstrated to correlate with improved efficacy in stroke reduction, there is still no consensus on what threshold constitutes an “acceptable” TTR from quality, effectiveness, and safety standpoints.
We determined the univariate effect of each variable on TTR separately and then completed a fully adjusted multivariate model. We used linear regression for our adjusted models using a generalized linear and latent mixed regression model adjusting for patient co-morbidities, INRMR, VHA parent station, and differential distance to quantify the independent effect of race on the conditional mean of TTR. We performed all analyses with SAS, version 9.3, (SAS Institute Inc; Cary, North Carolina) and STATA, version 11.0, (StataCorp; College Station, Texas).
Results
Of 523,675 patients identified with AF in FY04-FY12, 184,161 had newly diagnosed AF and met inclusion criteria ( Figure 2 ). Analysis of baseline characteristics ( Table 1 ) demonstrated that black patients had higher CHADS2 risk scores, ATRIA bleeding risk scores, Charlson, and Selim co-morbidity scores compared to all other races (p <0.0001). In particular, congestive heart failure, hypertension, and diabetes were significantly higher among blacks (p <0.0001).
| White | Black | Asian | Native Hawaiian/ Pacific islander | Native American | Multi-Racial | Missing | P-Value | |
|---|---|---|---|---|---|---|---|---|
| n=184,161 | 163,601 | 15,372 | 612 | 1,296 | 762 | 886 | 1,632 | |
| Age (mean) | 71±10 | 66±12 | 70±13 | 70±10 | 67±10 | 69±11 | 59±11 | <0.0001 |
| Gender, female (%) | 2,622 (2%) | 295 (2%) | 12 (2%) | 29 (2%) | 19 (2%) | 14 (2%) | 45 (3%) | <0.0001 |
| CHADS2 score | 1.6±1.2 | 1.8±1.3 | 1.6±1.2 | 1.7±1.2 | 1.5±1.3 | 1.7±1.3 | 0.9±1.0 | <0.0001 |
| Charlson comorbidity index | 1.7±1.6 | 2.1±1.7 | 1.6±1.5 | 1.8±1.6 | 1.8±1.6 | 1.9±1.6 | 1.0±1.2 | <0.0001 |
| Selim comorbidity index | 4.0±2.6 | 4.5±2.7 | 3.7±2.4 | 4.1±2.5 | 4.0±2.6 | 4.4±2.6 | 2.5±2.0 | <0.0001 |
| ATRIA Bleeding Risk Score | 3.0±2.2 | 3.5±2.4 | 3.2±2.5 | 3.0±2.3 | 2.7±2.3 | 3.2±2.3 | – | <0.0001 |
| Hypertension | 103,285(63%) | 11,482 (75%) | 380 (62%) | 886 (68%) | 444 (58%) | 620 (70%) | 708 (43%) | <0.0001 |
| CHF | 24,263 (15%) | 4,066 (26%) | 107 (17%) | 219 (17%) | 106 (14%) | 171 (19%) | 137 (8%) | <0.0001 |
| Diabetes | 50,562 (31%) | 5,768 (38%) | 186 (30%) | 488 (38%) | 284 (37%) | 316 (36%) | 348 (21%) | <0.0001 |
| Prior stroke/tia | 11,943 (7%) | 1,344 (9%) | 45 (7%) | 92 (7%) | 63 (8%) | 82 (9%) | 50 (3%) | <0.0001 |
| Dialysis | 2,326 (1%) | 776 (5%) | 27 (4%) | 32 (2%) | 27 (4%) | 28 (3%) | 11 (1%) | <0.0001 |
| Renal failure (eGFR ≤40), (n) | 13,943 (9%) (n=149,850) | 1,696 (12%) (n=14,038) | 75 (13%) (n=584) | 145 (12%) (n=1,197) | 68 (10%) (n=696) | 88 (10%) (n=825) | – | <0.0001 |
| Cardiovascular Medications | ||||||||
| Warfarin | 101,817 (62%) | 10,291 (67%) | 368 (60%) | 836 (65%) | 465 (61%) | 572 (65%) | 882 (54%) | <0.0001 |
| Any Anticoagulant | 102,742(63%) | 10.376 (67%) | 372 (61%) | 843 (65%) | 467 (61%) | 578 (65%) | 902 (55%) | <0.0001 |
| ASA | 40.901 (25%) | 6,719 (44%) | 238 (39%) | 379 (29%) | 234 (31%) | 294 (33%) | 408 (25%) | <0.0001 |
| Clopidogrel | 16,845 (10%) | 1,139 (7%) | 46 (8%) | 133 (10%) | 76 (10%) | 85 (10%) | 73 (4%) | <0.0001 |
| Any anti-platelet | 53,271 (33%) | 7,349 (48%) | 261 (43%) | 472 (36%) | 288 (38%) | 355 (40%) | 454 (28%) | <0.0001 |
| ACE inhibitor or ARB | 98,348 (60%) | 10,451 (68%) | 436 (59%) | 800 (61%) | 417 (60%) | 481 (63%) | 1,160 (53%) | <0.0001 |
| Diuretic | 87,236 (53%) | 10,139 (66%) | 306 (50%) | 734 (57%) | 406 (53%) | 523 (59%) | 693 (42%) | <0.0001 |
| Niacin or fibrate | 13,589 (8%) | 674 (4%) | 40 (7%) | 82 (6%) | 61 (8%) | 83 (9%) | 120 (7%) | <0.0001 |
| Statin | 100,309(61%) | 8,946 (58%) | 375 (61%) | 814 (63%) | 438 (57%) | 541 (61%) | 784 (48%) | <0.0001 |
| Class I antiarrhythmic | 3,288 (2%) | 193 (1%) | 15 (2%) | 25 (2%) | 12 (2%) | 20 (2%) | 55 (3%) | <0.0001 |
| Class III antiarrhythmic | 5,990 (4%) | 346 (2%) | 14 (2%) | 34 (3%) | 27 (4%) | 28 (3%) | 44 (3%) | <0.0001 |
| Alpha-blocker | 3,851 (2%) | 943 (6%) | 20 (3%) | 37 (3%) | 29 (4%) | 35 (4%) | 31 (2%) | <0.0001 |
| Beta-blocker | 110,679 (68%) | 11,311 (74%) | 409 (67%) | 907 (70%) | 512 (67%) | 621 (70%) | 1,104 (68%) | <0.0001 |
| Calcium channel blocker | 59,515 (36%) | 7,146 (46%) | 234 (38%) | 475 (37%) | 242 (32%) | 364 (41%) | 503 (31%) | <0.0001 |
| Digoxin | 34,127 (21%) | 3.039 (20%) | 111 (18%) | 264 (20%) | 167 (22%) | 176 (20%) | 322 (20%) | 0.0209 |
| Amiodarone | 15,184 (9%) | 1,550 (10%) | 56 (9%) | 125 (10%) | 67 (9%) | 90 (10%) | 137 (8%) | 0.0351 |
| Distance (miles) to closest VHA medical center | 40.5±44.2 | 23.2±27.9 | 20.5±28.8 | 39.4±42.8 | 49.5±48.6 | 35.8±58.1 | 47.9±52.0 | <0.0001 |
| Distance (miles) to closest VHA community clinic | 13.5±12.9 | 8.5±9.2 | 7.2±7.6 | 11.4±11.3 | 15.4±15.4 | 12.4±12.5 | 12.5±12.8 | <0.0001 |
| Δ of two distances (miles) | 27.0±41.4 | 14.7±25.2 | 13.3±26.2 | 28.1±40.7 | 34.1±44.1 | 23.4±56.4 | 35.4±50.0 | <0.0001 |
| Seen by cardiology within 90 days of AF (%) | 61,082 (37%) | 6,736 (44%) | 287 (47%) | 495 (38%) | 278 (36%) | 363 (41%) | 660 (40%) | <0.0001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


