Chapter 68 Q-T Interval, QT Dynamicity, and QT Variability
Q-T Interval
The Q-T interval includes the QRS complex; nevertheless, the entire Q-T interval is considered a measure of repolarization because the repolarization process already takes place during the QRS complex for the early activated regions of the myocardium.1,2 Because the process of depolarization and repolarization in the myocardium is sequential, certain regions are depolarized earlier and repolarization starts earlier in them compared with other regions. The most optimal approach to quantify the duration of repolarization represented as the Q-T interval is to obtain this measurement simultaneously from all 12 leads of the standard ECG.1,2 The Q-T interval should be measured from the earliest onset of the QRS complex in any lead to the latest offset of the T wave in any lead, thereby providing a total measure of repolarization duration.1–3 This approach is exercised in some automatic algorithms incorporated in ECG machines, but manual measurements of the Q-T interval usually rely on a single lead (most often lead II), which under-represents Q-T interval duration. In the case of a flat T wave in lead II, lead V5 is most frequently chosen as an alternative because electrical forces of V5 resemble those of lead II.
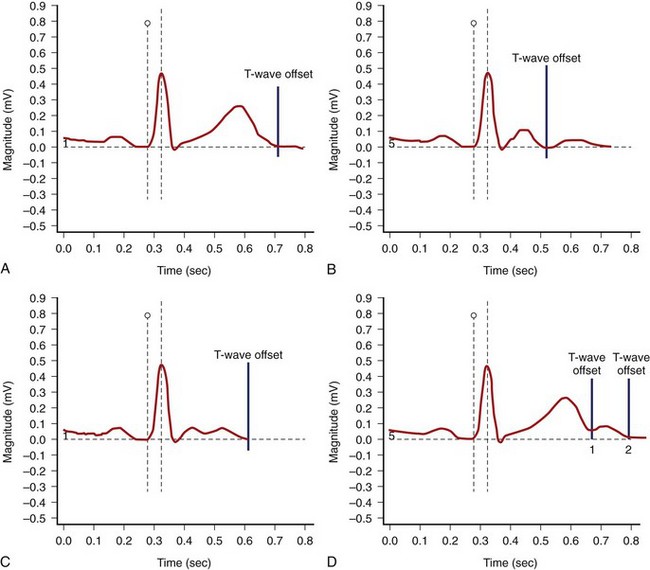
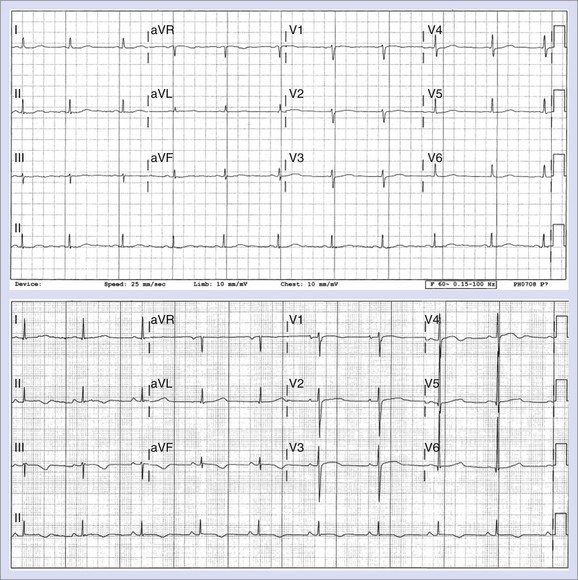
The end of the T wave is defined as the point at which the descending or ascending arm of the T wave terminates, usually at the level of the isoelectric line, or as the nadir between the T and U waves.1–4 When the T and U waves merge or when the T wave has a bifid appearance with two components, careful inspection of repolarization duration in all 12 leads may be helpful in determining the proper approach to quantify the Q-T interval.5,6 In some cases, a QTU duration should be reported, with acknowledgment that the measurement includes a second component that may be part of the T wave or superimposed U wave (Figure 68-1). In case of repolarization morphologies, with the presence of a second component of the T wave or the U wave, some investigators suggest using the rule that the U wave usually should be at least 150 ms behind the peak of the T wave, whereas closer deflections may be considered the second component of the T wave.5,6 However, no systematic data are available to validate this rule.
The Q-T interval duration is different among standard 12 leads, and QT dispersion has been measured in numerous studies as a possible marker of arrhythmogenic conditions.7,8 However, because of the conceptual and methodologic limitations of QT dispersion, this method has not been approved as a standard tool in clinical practice or drug studies. Inter-lead differences in repolarization morphology, rather than just Q-T interval duration, seem to better reflect the complexity of the repolarization process.9–11 Inter-lead differences in repolarization morphology could be quantified by using various methods, including principal component analysis and area-based analysis of the repolarization segment.9–11 Principal component analysis of the repolarization segment allows quantifying the length (λ1) and width (λ2) of the T-wave loop.9,10 The roundness of the T-wave loop in its preferential plane (λ2/λ1) has been considered an index of increased T-wave complexity.
QTc Formulas
Bazett’s formula, describing a curvilinear association between the Q-T and R-R intervals (QTc = QT/[RR1/2]), is most frequently used.12 Fridericia’s formula (QTc = QT/[RR1/3]), or the cubic root formula, is used in studies evaluating the effects of medications on the Q-T interval.13 Both formulas adjust the Q-T interval to reflect repolarization duration at a heart rate of 60 beats/min. Bazett’s QTc formula has limitations of overestimating the repolarization duration at fast heart rates and underestimating it at slow heart rates. Fridericia’s formula causes less misjudgment; however, clinicians are not comfortable using this formula on a daily basis because of the need for a cubic root equation and, more importantly, because of limited clinical data on normal values and the diagnostic and prognostic significance of this measurement. Other QTc formulas have been proposed (Box 68-1).12–17
Box 68-1 Examples of QT Correction Formulas
In drug studies, QTc corrected by Fridericia’s formula is the most frequently used, but the analysis of QT-RR regression based on the subject-specific QT-RR relationship plots is attracting increasing interest.18,19 The RR bin method is yet another approach that measures the Q-T interval in absolute value for matched R-R intervals for recordings off and on drugs without the need for correction formulas.20,21 Both methods are particularly valuable when evaluating drugs affecting heart rates.
Reference QTc Values
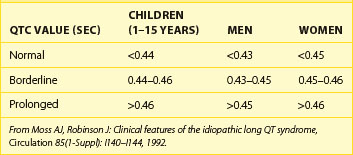
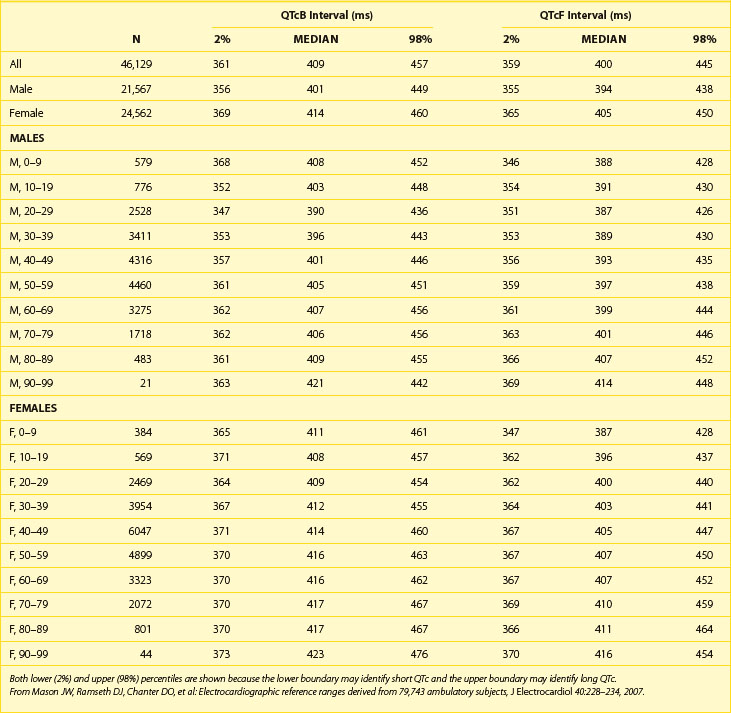
Table 68-1 shows abnormal QTc values (using Bazett heart rate correction) by age and gender. Women and children have a longer duration of repolarization than men.22 Gender-related differences in QT are predominantly caused by a shorter Q-T interval duration in men than women at age 15 to 55 years, not because of Q-T interval prolongation in women.16 Data from a large cohort of more than 46,000 healthy individuals participating in pharmaceutical QT studies, as reported by Mason et al, are fully supportive of the initial ranges of QTc values reported by Moss et al.22,23 Table 68-2 shows values for QTcB (Bazett corrected QTc) and QTcF (Fridericia corrected QTc) for age-specific and gender-specific groups from this large cohort. In general, the 98th percentile value for QTcF is approximately 10 ms shorter than the respective value for QTcB for a given age and gender group. Factors influencing gender differences in QTc duration may include slower heart rate in men, different density of potassium ion channels in the male myocardium versus the female myocardium, effects of female hormones contributing to longer Q-T interval duration in women, and possibly the effect of male hormones contributing to the shorter Q-T interval duration in men. Differences by age also exist, with healthy older adults demonstrating a longer QTc duration compared with younger individuals.
T-Wave Morphology
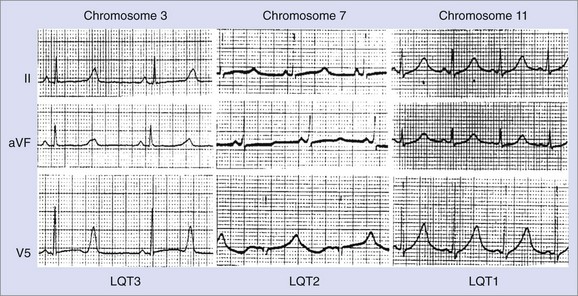
Changes in T-wave morphology are frequently observed in association with myocardial ischemia, hypertrophy, overload, and genetic alterations in repolarization. LQTS shows variations of T-wave morphology, which could be related to the type of affected ion channel and the magnitude of ion channel dysfunction as well as other factors, such as age, heart rate, and the status of the autonomic nervous system. Specific genetic types of LQTS could be associated with distinct ECG patterns of repolarization.24,25 Figure 68-2 shows specific patterns associated with distinct genetic types of the disorder: LQT1, characterized by wide, broad-based T waves; LQT2, usually showing low-amplitude and frequently notched T waves; and LQT3, characterized by a relatively long ST segment followed by a peaked, frequently tall T wave. In the Rochester ECG Core Lab, we use a 3-digit code (Box 68-2), which is a classification to characterize the T wave and the Q-T interval visually.26 The first digit represents a description of T-wave morphology, the second digit provides information about its polarity, and the third digit indicates whether the U wave is present or whether the TU complex should be measured because of the presence of the second component of the T wave or the U wave merging with the T wave. Recognizing abnormal T-wave morphology may be particularly useful in diagnosing patients with borderline QTc duration (420 to 470 ms). In such patients, the presence of an abnormal (notched, flat) T wave may indicate the possibility of LQTS (Figure 68-3).
Box 68-2 Coding of QT/T-Wave Morphology
From Zareba W, Moss AJ, Rosero SZ, et al: Electrocardiographic findings in patients with diphenhydramine overdose, Am J Cardiol 80:1168–1173, 1997.)
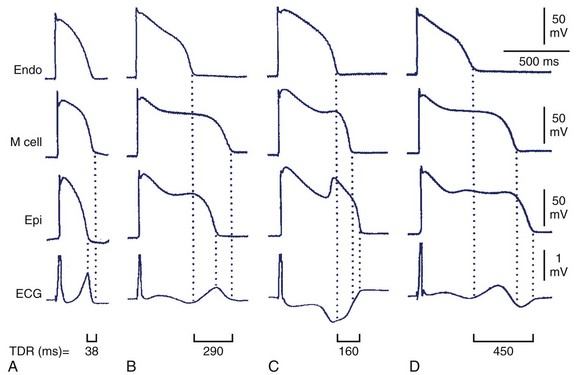
Decreased T-wave amplitude with an increased presence of notches may be observed in drug-induced changes in repolarization. Drug-induced changes in T-wave morphology reflect changes in the transmural gradient of repolarization with propensity to arrhythmogenesis (Figure 68-4).27,28 Therefore the identification of drug-induced changes in repolarization morphology in clinical studies should always trigger attention because those changes may indicate a propensity to proarrhythmia.
Drug-Induced Q-T Interval Prolongation
Several cardiac and noncardiac medications may cause QTc prolongation and cause torsades de pointes (TdP) (Table 68-3).29 They usually block the IKr current, whereas others exert such action if administered together with a drug affecting the function of the cytochrome P-450 enzymatic system. A number of quinidine-induced sudden cardiac deaths (SCDs) and, subsequently, the results of the Cardiac Arrhythmia Suppression Trial (CAST) brought further attention to the proarrhythmic effects of antiarrhythmic drugs.30 The antihistamine drug terfenadine was recognized as the first noncardiac medication causing TdP and SCD.31 Terfenadine blocks the IKr current (as well as the Na+ current and the L-type calcium channel), causing a mean 6-ms QTc prolongation, which should not have clinical implications. Cardiac events were reported in patients taking terfenadine, almost exclusively when used in combination with other medications (ketoconazole, antibiotics) also metabolized by the same enzymatic system of the cytochrome P-450 3A4. Such a combination causes an increase in plasma concentration of terfenadine because of inhibition of its metabolism by a concomitantly administered drug with subsequent substantial QTc prolongation (>60 ms in the majority of reported cases) and TdP. Among noncardiac drugs, several antipsychotic drugs block the IKr current and cause QTc prolongation, and some of them were reported to be associated with SCD.32
Table 68-3 Drugs That Prolong the Q-T Interval
| CATEGORY | DRUGS |
|---|---|
| Antihistamines | Astemizole, terfenadine |
| Anti-infectives | Amantadine, clarithromycin, chloroquine, erythromycin, grepafloxacin, moxifloxacin, pentamidine, sparfloxacin, trimethoprim-sulfamethoxazole |
| Anti-neoplastics | Tamoxifen, arsenic trioxide |
| Anti-arrhythmics | Quinidine, sotalol, procainamide, amiodarone, bretylium, disopyramide, flecainide, ibutilide, moricizine, tocainide, dofetilide, ranolazine, vernakalant, and dronedarone |
| Anti-lipemic agents | Probucol |
| Calcium channel blockers | Bepridil |
| Diuretics | Indapamide |
| Gastrointestinal agents | Cisapride |
| Hormones | Fludrocortisone, vasopressin |
| Anti-depressants | Amitriptyline, amoxapine, clomipramine, imipramine, nortriptyline, protriptyline |
| Anti-psychotics | Chlorpromazine, haloperidol, perphenazine, quetiapine, risperidone, sertindole, thioridazine, ziprasidone, doxepin, methadone |
Updated information on QT prolonging drugs can be found at www.qtdrugs.org.
From Zareba W: Drug induced QT prolongation, Cardiol J 14:523–533, 2007.
Box 68-3 lists common factors predisposing to QTc prolongation and TdP.29 Females with faster resting heart rates and a longer QTc interval compared with men are particularly prone to drug-induced TdP. Older patients and those with underlying heart diseases (cardiomyopathies) or electrolyte abnormalities, especially hypokalemia and bradycardia, are particularly at an increased risk for drug-induced QTc prolongation and TdP.