Pulmonary Disorders and Pregnancy
INTRODUCTION
Nearly six million pregnancies are reported in the United States every year. A number of characteristics of the pregnant population appear to be changing over time. For example, women are becoming pregnant later in their reproductive lives. In addition, with advances in medicine and assisted reproductive technology, women with chronic medical conditions are now able to become pregnant. Consequently, pregnant women are now older and have more comorbid conditions than in past generations. Given the physiologic changes that occur during gestation, pregnancy can be an important stressor to chronic medical conditions. Notably, the health of the pregnancy may be a window into the mother’s long-term health.
Labor and delivery constitute a major hemodynamic and respiratory challenge; hence, women with both acute and chronic conditions need to be managed by a multidisciplinary healthcare team, which may include a pulmonologist. This multidisciplinary team should address medical and obstetrical concerns, along with risks and benefits of anesthesia and analgesia, thereby ensuring appropriate management and anticipation of potential complications.
This chapter addresses core pulmonary and cardiovascular physiologic concepts before consideration of important, common clinical entities related to diagnosis and management in pregnancy.
THE PHYSIOLOGY OF PREGNANCY
While the normal physiologic changes of pregnancy are extensive, several areas are of central importance and are considered below. These include changes in respiratory and cardiovascular physiology, normal physiologic developments during labor and delivery, and determinants of fetal oxygenation and ventilation.
 RESPIRATORY PHYSIOLOGY
RESPIRATORY PHYSIOLOGY
During pregnancy, the respiratory system undergoes changes which range anatomically from the nasopharynx to the lungs.
The effects of increasing levels of estrogen on the nasal mucosa include edema, hyperemia, and glandular hypersecretion. The result is gestational rhinitis, which typically occurs in the last few weeks of pregnancy.
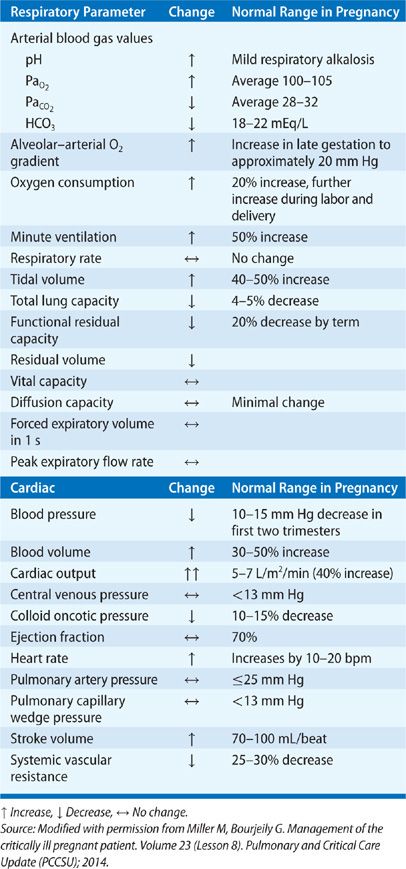
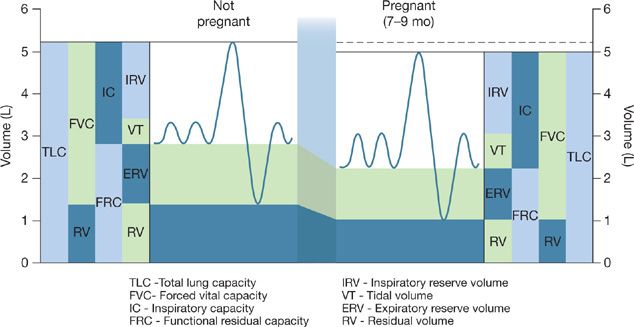
The subcostal angle of the rib cage and the circumference of the chest wall increase due to the effects of relaxin.1 The diaphragm moves cranially about 4 to 5 cm.2 These changes occur to accommodate the growing uterus. Diaphragm excursion does not decrease in pregnancy, despite the changes in chest wall configuration. However, due to the higher resting position of the diaphragm, the decreased downward pull of the abdomen, and the aforementioned chest wall changes, functional residual capacity (FRC) decreases by 20% to 30% by late gestation3 and declines further in the supine position (Table 97-1).4 The reduction in FRC may be somewhat attenuated when measured using body plethysmography,5 likely due to the effect of early airway closure during tidal breathing and air trapping.6 Inspiratory capacity increases and, therefore total lung capacity (TLC) is not significantly changed (Fig. 97-1).7
Figure 97-1 Changes in lung volumes in pregnancy. (Reproduced with permission from Hegewald MJ, Crapo RO. Respiratory physiology in pregnancy. Clin Chest Med. 2011;32(1):1–13.)
Despite a reduction in FRC and reduced nasopharyngeal caliber, air flow rates and airway resistance do not change in pregnancy, since pregnancy is associated with bronchial smooth muscle relaxation.8 While the increased cardiac output of pregnancy (see Section “Cardiac Physiology”) may increase diffusion capacity, the associated anemia of pregnancy offsets the effect. DLCO is higher in the first trimester (after correcting for alveolar volume and hemoglobin), but it may decline later in pregnancy, reaching a nadir at about 24 to 27 weeks of gestation.9
Progesterone is a respiratory stimulant; consequently, minute ventilation in pregnancy increases by about 50% due to an increase in tidal volume. Although some studies suggest a progressive increase in minute ventilation during the course of pregnancy,3 most studies show a sharp increase in the first trimester, with minimal rise after that.10,11 The ratio of dead space to tidal volume (VD/VT) is unchanged in pregnancy, suggesting that dead space is actually also increased. Although the increase in cardiac output seen in pregnancy, coupled with resulting improved perfusion of the lung apices, would suggest that a reduction in dead space should occur, many studies suggest the opposite effect.12,13
Arterial oxygen tension increases in pregnancy to about 100 to 105 mm Hg, thereby facilitating oxygen transfer across the placenta.13 Carbon dioxide tension decreases in pregnancy to 27 to 32 mm Hg, with a resultant increase in bicarbonate excretion and elevation in arterial pH to approximately 7.42 to 7.46. Both PaCO2 and HCO3 are mildly lower in the third trimester compared to the first trimester.14 The chronic respiratory alkalosis of pregnancy causes a rightward shift of the oxyhemoglobin dissociation curve, facilitating oxygen transfer across the placenta.
 CARDIAC PHYSIOLOGY
CARDIAC PHYSIOLOGY
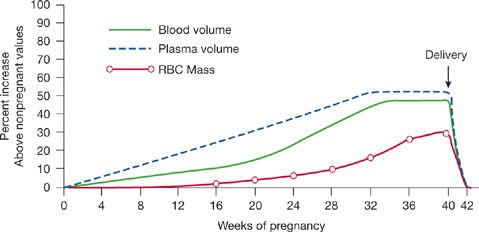
In parallel with growth of the fetus and the accompanying increase in maternal oxygen consumption, pregnancy is accompanied by profound cardiovascular changes. Plasma volume and cardiac output progressively increase throughout gestation and are approximately 30% to 50% higher15 at term (Fig. 97-2).16 Heart rate also increases by 20 to 30 beats per minute. Systemic vascular resistance decreases, with a resultant drop in blood pressure in the first half of pregnancy. Despite the increase in cardiac output, pulmonary artery pressures do not change significantly17 due to a decrease in pulmonary vascular resistance (PVR). Oxygen consumption is increased approximately 20% above prepregnancy levels; the rate of increase in oxygen consumption is faster in pregnant women compared to nonpregnant women performing weight-bearing exercises.18
Figure 97-2 Physiologic changes in blood volume, plasma volume, and erythrocyte mass in pregnancy.
 LABOR AND DELIVERY
LABOR AND DELIVERY
Labor and delivery are associated with additional respiratory and cardiac physiologic changes. Pain, due to contractions and anxiety, leads to increases in oxygen consumption and minute ventilation. Increased minute ventilation leads to further reduction in PaCO2 which, in cases of tenuous placental reserve, may reduce placental perfusion. In addition, in women with poor respiratory reserve, such as those with poorly controlled asthma or restrictive pulmonary physiology, the additional increase in minute ventilation may lead to respiratory decompensation and respiratory failure. Pain control may help minimize these effects. However, the use of systemic opiates can lead to respiratory depression, whereas targeted regional anesthesia – thereby avoiding an effect on respiratory muscles – may prevent these adverse effects. Physiologic changes occurring in pregnancy quickly reverse in the postpartum period.
Cardiovascular changes in labor, delivery, and the postpartum period include an additional 10% to 15% increase in cardiac output in early labor, with further increases of up to 50% in the second stage of labor.19 The changes are mediated by autotransfusion, in which 300 to 500 mL of blood is shunted into the systemic maternal circulation with every uterine contraction,20 and by pain and anxiety.21,22 Analgesia attenuates the changes. In addition, in the postpartum period, relief of aortocaval compression by the uterus results in an increase in venous return; autotransfusion from the contracting uterus further increases cardiac output. Women with underlying cardiac or pulmonary vascular disease may not tolerate the physiologic changes of labor and delivery. Although scheduled Cesarean-section deliveries may appear to be an attractive option for women with chronic pulmonary or cardiac disease (since they may eliminate the pain and anxiety of contractions), Cesarean-section deliveries are unlikely to improve outcomes, since autotransfusion occurs with both vaginal and operative deliveries. Furthermore, the immediate fluid shifts that occur in the postpartum period are similar with both vaginal and Cesarean-section deliveries. In addition, operative deliveries are associated with higher rates of surgical and medical complications compared with vaginal deliveries.
 FETAL OXYGENATION AND VENTILATION
FETAL OXYGENATION AND VENTILATION
Uterine blood flow constitutes about 17% of the total cardiac output.23 The placental vessels are low-resistance, high-capacitance vessels that function at maximal capacity at baseline. Therefore, when a hemodynamic insult occurs, the placental circulation cannot further vasodilate.
Supine positioning in late pregnancy may be associated with significant reduction in cardiac output and may alter the uterine circulation. Fetal oxygenation, like oxygenation of maternal organs, depends on maternal cardiac output, maternal oxygenation, and the fetal oxyhemoglobin dissociation curve. In addition, characteristics of the placenta affect diffusion of oxygen across this organ.
The fetus displays many protective mechanisms for maintaining adequate oxygenation. For example, fetal hemoglobin, which lacks beta chains, is less sensitive to the effects of 2,3-DPG than is adult hemoglobin. Consequently, the fetal oxyhemoglobin dissociation curve is shifted to the left, reflecting an increased oxygen affinity. The fetus also appears to have the ability to shunt blood to vital organs and to decrease oxygen consumption during periods of hypoxia. Despite these protective mechanisms, placental health has an important role in effecting oxygen transport to the fetus.24
Clinical conditions that cause a reduction in cardiac output or uterine blood flow (e.g., shock, use of vasoconstricting drugs), oxygen content (e.g., anemia, hypoxemia), oxyhemoglobin saturation (e.g., severe alkalosis, presence of carboxyhemoglobin), or placental perfusion may result in poor fetal oxygenation. Human studies on the optimal maternal oxygen level needed to achieve favorable fetal oxygenation are scarce. Based on available animal data, experts recommend that maternal oxygenation be maintained above a PaO2 of 70 mm Hg and an oxygen saturation of greater than 95%.
Finally, a gradient for CO2 of approximately 10 mm Hg between fetal and maternal circulations exists (higher level in the fetus), facilitating CO2 clearance from the fetal circulation. This fact may influence how best to use permissive hypercapnia in patients who might benefit from this ventilatory strategy. Fetal hypercapnia results in rapid fetal acidemia, increases in intracranial pressure, and a rightward shift of the oxyhemoglobin dissociation curve.
DIAGNOSTIC IMAGING OF THE PREGNANT WOMAN
Use of ionizing radiation is a major concern for most clinicians evaluating a pregnant patient. Risks that need to be considered when ordering an imaging study that involves the use of ionizing radiation are the risk of malignancy and the risk of teratogenicity.
The risk of oncogenicity follows a stochastic effect (nonthreshold effect), suggesting that the risk of malignancy may occur even at low levels of exposure. Based on atomic bomb survivor data, the risk of malignancy following high-dose exposures is indisputable; however, the risk following low doses associated with diagnostic testing is more controversial. This risk is not evenly distributed, as recent data suggest a lower risk of cancer when exposure occurs in utero compared to exposure in childhood.25
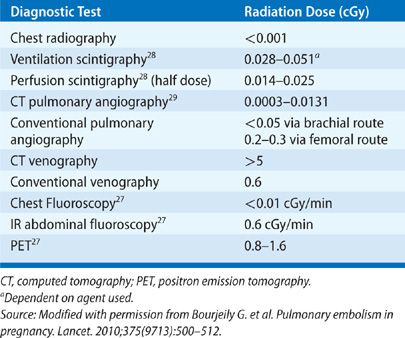
The risk of teratogenicity, however, does follow a threshold effect. Based on animal and human studies of radiation exposure, a threshold has been determined for congenital malformations and miscarriage. For diagnostic tests resulting in a fetal exposure of 10 cGy or less, the risk of radiation to the embryo is very small when compared to the spontaneous risk. More conservative opinions suggest aiming for a maximum exposure of 5 cGy. With the exception of computed tomography venography, radiation exposure from imaging procedures used for the workup of a patient with a suspected or diagnosed respiratory disorder does not exceed 5 cGy (Table 97-2).26 As the risk of radiation below 10 cGy is miniscule, medical care of the mother takes priority over the risks of diagnostic radiation exposure to the fetus.27
Another consideration in diagnostic imaging studies includes use of contrast agents. Iodinated contrast media are small molecules that are known to cross the placenta and carry a potential risk of thyroid dysfunction in utero. However, in a study of 350 women who received iodinated contrast in pregnancy, none of the babies born had any significant thyroid dysfunction.30 The study was, however, performed in the northeast United States, an area where iodine deficiency is rare. Use of iodinated contrast is, therefore, certainly justifiable. However, iodine reserve, timing of the study in pregnancy, and maternal renal function should be considered. Gadolinium is usually used with nonionizing radiation imaging studies and is known to cross the placenta.31 The effect of gadolinium on the growing fetus is unknown, as human safety data are not available. Hence, use of gadolinium should be reserved for cases where benefit outweighs the potential unknown risk of gadolinium exposure.
Diagnostic studies involving radionuclides expose the fetus to radiation differently than external radiation. Given that radionuclides are excreted by the kidneys and may pool in the bladder, continuous exposure to the fetus may occur after the study has been completed.
PHARMACOTHERAPY CONSIDERATIONS IN THE PREGNANT PATIENT
Over-the-counter drugs and prescription medications are frequently used during pregnancy. Although a number of drugs have been clearly shown to be either teratogenic or safe in pregnancy, insufficient safety data exists for most. Most data on safety for a given drug in pregnancy are based on animal studies, case reports or series, case-control studies, or pregnancy registries. Prospective studies evaluating safety are scarce. Establishing risk is challenging, since a baseline risk of malformation exists for any pregnancy. To clearly define a teratogenic risk, a recurrent syndrome of abnormalities needs to be demonstrated. In addition, in many cases it may be hard to tease out the effect of the medication from the effect of the disease. Justification of randomization to treatment or placebo groups during pregnancy is challenging. An informed decision by the patient, formulated in the context of discussion with the physician, is key. Integral to the discussion is the patient’s understanding of the limitations of the available data and the intricacies of pharmacotherapy in pregnancy.
Placental transfer of medications and their risks depend on maternal drug metabolism, drug molecular size, drug ionization, the agent’s lipid solubility, and maturity of the fetal target organ. As a general rule, most drugs cross the placenta. Although most consider the period beyond embryogenesis a safe timeframe to use medication, it is important to recall that the fetal nervous system continues to develop throughout gestation. For this reason, fetal neurologic and behavioral development and function of various organs, such as thyroid or kidney, may be affected by medications administered after the first trimester. Physiologic changes of pregnancy, such as increased plasma volume, increased glomerular filtration rate, and hypoproteinemia may affect medication bioavailability.
The categories of drug safety in pregnancy used by the US Food and Drug Administration (FDA) have several well-established shortcomings. For example, since teratogenicity is not species specific, supportive evidence from human and animal data should not be given equal weight. The risk of drugs in a given category may not be comparable. For instance, drugs in category C either have been shown to demonstrate evidence of adverse effects in animals or lack available animal data. In addition, adverse fetal effects are not all equal, and a mild reduction in fetal weight should not be considered equivalent to a major congenital anomaly. Finally, the FDA categorization does not take into account the risk of the untreated disease. Therefore, the decision for using a drug should be based on principles of efficacy and safety and should encompass consideration of medication risk versus risk of untreated disease. For example, use of a category C drug in a life-threatening situation may be reasonable, whereas use of a category B drug for a self-limited condition, such as an upper respiratory infection, may not.
Breastfeeding is associated with significant benefits to newborns, such as a lower risk of obesity, diabetes, and cardiovascular disease, among others,32–34 and consideration of drug passage into breast milk is an important consideration. A variety of factors influence drug passage to the neonate. For example, drugs with no oral absorption by the neonate are unlikely to cause systemic effects. Drug levels in breast milk are proportional to those in the maternal serum. Hence, breast milk drug levels vary with timing of maternal drug intake. Drugs with the highest protein binding and lowest lipid solubility are preferable for breastfeeding women.35 Table 97-3 summarizes data on commonly used drugs in pregnancy and lactation.
INTERVENTIONAL PROCEDURES IN PREGNANCY
Indications for interventional procedures in pregnancy are no different than in the nonpregnant population. In the general population, most interventional procedures are not delayed if they are anticipated to be of clinical importance in patient management. Similar logic should be applied in pregnancy. As with any intervention, the risk of the intervention should be weighed against the risk of an inappropriate diagnosis or untreated disease. For example, the risk of bronchoscopy in pregnancy may be related to the procedure itself, as well as to the sedatives used during the procedure. Medication effects may be even more pronounced in the pregnant patient, given the altered physiology of pregnancy, including drug distribution, response to sedatives, and decreased airway patency. However, the risk is usually minimal.
In pregnant women, an upper airway evaluation should be performed prior to every procedure, given the higher risk of intubation failure in pregnancy. Risk of aspiration may also be increased because of a decrease in lower esophageal and increase in intra-abdominal pressures. To minimize the risk of hypotension and hemodynamic instability during procedures performed in the second half of pregnancy, patients should be positioned supine with a left tilt to relieve the compression of the inferior vena cava by the gravid uterus.
Given the risk of hypoxemia with bronchoscopy,41 especially when bronchoalveolar lavage is performed,42 oxygenation should be monitored routinely and supplemental oxygen administered as needed to keep oxygen saturation above 95%. Fetal monitoring should be discussed with the obstetric team; however, in general, fetal monitoring prior to 28 weeks of gestation can be unreliable and difficult to interpret, while monitoring prior to 24 weeks is likely unnecessary.43 Since pregnant women may more quickly develop hypercapnia and hypoxemia than nonpregnant women,44 smaller doses of sedation might be considered. Midazolam, meperidine, fentanyl, and lidocaine have not been reported to be associated with congenital malformations.45
Fluoroscopy during bronchoscopy can be used in pregnancy, given the low dose of radiation associated with its use (Table 97-2)26 and the fact that the fetus is not in the radiation field. A discussion with the institution’s medical physicist can help keep radiation exposure to a minimum.
Pleural procedures can also be performed in pregnancy, but providers should keep in mind the potential 4 to 5 cm cranial displacement of the diaphragm during pregnancy. Ultrasound guidance may help minimize risks of the procedure.
SMOKING DURING PREGNANCY
Estimates are that 22% of women of reproductive age in the United States smoke. Data from Europe and the United States show that 10.7% to 13.1% of fetuses are exposed to maternal smoking. Although about half of women smokers quit during pregnancy, many start smoking again after delivery. In 1993, the estimated annual cost of maternal conditions attributable to smoking in pregnancy, including the protective effect against preeclampsia, was between $135 and $167 million.46,47
Smoking is associated with adverse fetal outcomes. Various mechanisms may be responsible. Placentas from smokers have reduced capillary volume and increased thickness of villous membranes.48–50 Carboxyhemoglobin is cleared slowly from the fetal circulation and competes with fetal oxyhemoglobin. Amniocytes from smokers have an increased incidence of structural chromosomal abnormalities compared with nonsmokers.51 In addition, nicotine promotes platelet activation, impairs estrogen synthesis, and can directly impair lung development due to interaction with nicotinic acetylcholine receptors.
Smoking is associated with placental abnormalities, premature rupture of membranes, decreased thyroid function, psychiatric illness, reduced fertility, and conception delay,52–56 as well as decreased production of milk volume, and lower rates and shorter duration of breastfeeding.57 Prospective58 and retrospective studies59 confirm an association of fetal growth restriction with smoking during pregnancy, even in the first trimester.60 Both active and secondhand smoke exposures are associated with increased risk of first trimester pregnancy loss.61
Babies born to pregnant smokers are also at risk for preterm birth.62 The association of smoking with a risk of congenital anomalies63 is a complex issue and may be closely related to maternal genotype. Maternal smoking during pregnancy is also associated with adverse cognitive and behavioral outcomes in the offspring, such as low scholastic achievement, attention deficit and hyperactivity disorder, conduct disorder, regular smoking,64 wheezing, and asthma.65 Childhood exposure to secondhand smoke is associated with upper and lower respiratory tract infections, ear infections, sudden infant death syndrome (SIDS), and asthma.66–68
Smoking cessation at any point during pregnancy is likely associated with some benefit.69 Pregnancy offers a unique opportunity for smoking cessation, given the mother’s motivation for fetal well-being and her frequent contact with healthcare providers. Women should be screened for secondhand smoking exposure, as well as potential abuse of other substances and comorbid psychiatric illness. Women should also be followed for smoking abstinence into the postpartum period, given the high rate of smoking relapse.
Pharmacotherapy can be used in pregnancy, but data regarding efficacy and safety are scarce. The US Preventive Services Task Force states that the use of pharmaceuticals has not been sufficiently evaluated to determine their safety or efficacy in pregnancy.70 Data on nicotine replacement therapy shows conflicting evidence with regard to safety.71,72 Many trials have been halted in the United States because of either adverse effects or failure to demonstrate effectiveness.73,74 The Danish National Birth Cohort of 72,761 women showed no significant association between type or duration of nicotine product use and birth weight.75 Overall, the risk from various chemicals in cigarette smoke likely outweighs the small risk of possible weight reduction from nicotine pharmaceuticals.
Although animal data on the safety of use of varenicline in pregnancy are available, human data are lacking. Varenicline is likely transferred through breast milk; however, data are not available. Bupropion has not been associated with an increase in congenital defects in experimental animal studies. However, pregnancy registry data for bupropion calls attention to a possible association with congenital heart defects.76 Although bupropion is passed to the fetus in breast milk, based on measurement of infant plasma levels, the dose transmitted is unlikely to be clinically significant.
MANAGEMENT OF ASTHMA DURING PREGNANCY
Asthma is the most common respiratory disease of pregnancy, affecting 4% to 8% of all pregnancies in the United States and almost 12% of pregnancies in the United Kingdom and Australia. Asthma may improve, worsen, or remain unchanged in pregnancy.77 Predictors of disease course include prepregnancy asthma severity, race, presence of obesity, compliance with medication use, and patient difficulties in accessing antenatal care. Factors contributing to modifications of disease severity include a number of pregnancy-associated conditions: higher prevalence of gastroesophageal reflux disease,78 nasal congestion,79 alterations in immunity, and hormonal factors.80 The rate of asthma exacerbations is increased between 17 and 32 weeks of gestation.77,81 Improvement in the last few weeks of pregnancy may be related to significantly higher cortisol levels compared with preconception levels.
Poorly controlled asthma may negatively impact some maternal and fetal outcomes. In the largest study performed to date, asthmatic women were more likely to have pregnancies complicated by miscarriage, antepartum and postpartum hemorrhage, anemia, or depression.82 In a retrospective cohort study performed in 12 clinical centers in the United States, asthmatics experienced increased risk of preeclampsia; gestational diabetes; preterm births, including spontaneous preterm birth; and induced preterm birth.83 A systematic review of nine studies has shown an increased risk of preterm birth, small for gestational age and low birth weight if the mother has asthma.84 Babies born to asthmatic mothers may be more likely to have congenital anomalies.85,86
Since pregnancy does not affect air flow rates, reductions in peak expiratory flow rate (PEFR) should prompt quick medical evaluation. In a multi-institutional prospective study, lower forced expiratory volume in 1 second (FEV1), but not symptom control, was associated with adverse perinatal outcomes.87 These data may be related to the fact that, given the high prevalence of physiologic dyspnea in pregnancy, symptom interpretation both by providers and patients may be difficult without objective measures; hence, the need for close physiologic monitoring is apparent.
Most medications used to treat asthma are safe for use in pregnancy (Table 97-3). An important message to patients is that good asthma control is the main goal, and the risk of medication use is lower than the risk of adverse outcomes from untreated or poorly treated disease. Recent evidence from a double-blind, randomized, controlled trial suggests that pregnant women managed according to a validated treatment algorithm based on measurements of exhaled nitric oxide (FeNO) had fewer exacerbations compared with those treated according to a symptom-based approach.88
TABLE 97-3 Drug Safety in Pregnancy and Lactation
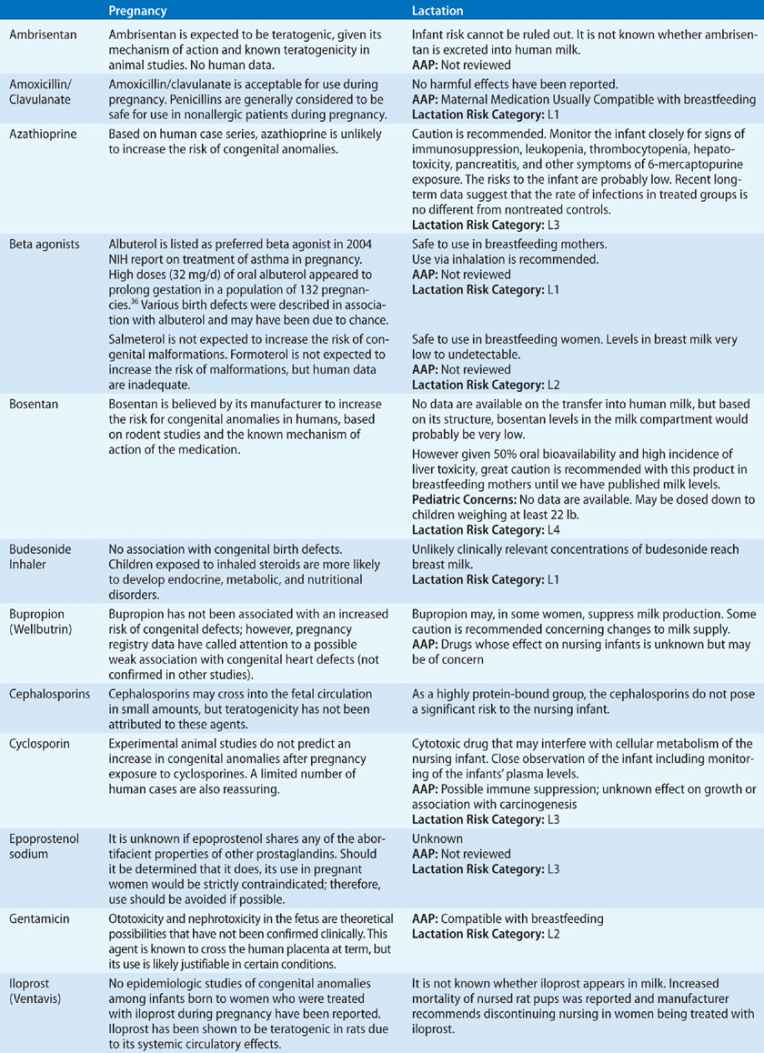
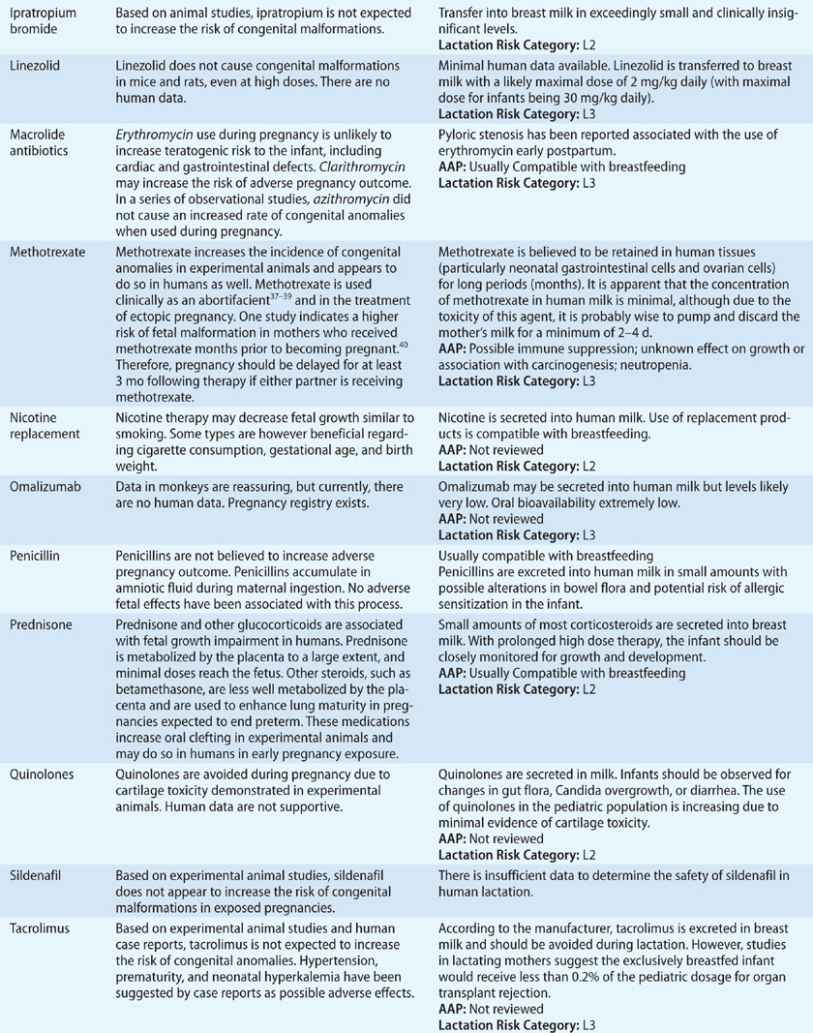
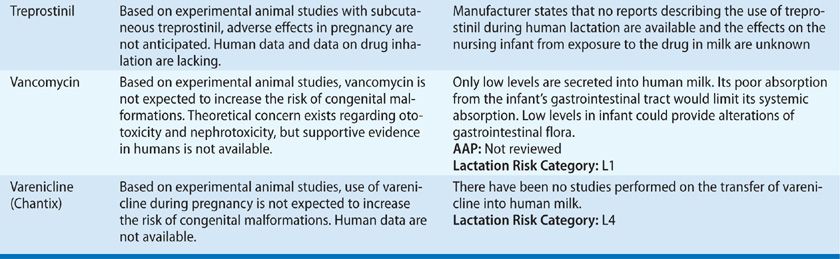
Patients with more severe disease, or those who suffer an exacerbation close to term, require a detailed labor and delivery plan. Stress dosing with corticosteroids during labor should be considered in patients who have been on prolonged courses of systemic steroids during the pregnancy. Patients with active symptoms or more severe asthma may benefit from regional anesthesia aimed at reducing minute ventilation and oxygen consumption and preventing hyperinflation. If general anesthesia is considered, ketamine and halogenated anesthetics are preferred. Oxytocin and prostaglandin E2 are safe to use. However, other prostaglandins used for induction of labor and morphine and meperidine should be avoided, since they may cause worsening bronchospasm.
CYSTIC FIBROSIS AND PREGNANCY
Cystic fibrosis (CF) impacts fertility in both men and women. Women with CF have various reproductive system abnormalities. Since the CF transmembrane conductance regulator (CFTR) is present in reproductive organs, cervical mucus is thicker and more tenacious, leading to obstruction of the cervical os and impaired sperm penetration. Despite these changes, fertility occurs in women with CF, and affected women should be counseled regarding contraception. Women with higher body weight and higher FEV1 are more likely to desire and achieve pregnancy.89 Genetic screening of both mother and father can help assess the risk of CF in the offspring. Preimplantation genetic diagnosis can be performed during assisted reproduction and may help detect embryos affected by the disorder.
In two series reporting on 72 and 90 pregnancies in patients with CF, the need for intravenous antibiotic therapy was 65% to 77%, and higher rates of hospitalization compared to nonpregnant patients were reported.90,91 FEV1 may decline during pregnancy in CF, and then improve in some patients following delivery.92 However, in larger case-controlled series, the FEV1 decline appears similar to that experienced by nonpregnant patients with CF.90,93 Reduced FEV1 appears to be a predictor of adverse outcomes in pregnancy in CF,91 including early mortality following delivery. Other predictors of decreased survival include maternal colonization with Burkholderia cepacia and pancreatic insufficiency.
Increased energy expenditure in pregnancy makes weight gain more challenging. Glucose intolerance and gestational diabetes occur as a result of decreased insulin production, impaired insulin sensitivity, and increased hepatic glucose production.93,94 Multiple studies have shown an increased risk of preterm delivery,90,95 possibly related to altered lung function. The risks of fetal demise and birth defects are likely similar to controls.90,91,93
Azithromycin, penicillins, and cephalosporins are safe to use in pregnancy. In animal studies, use of fluoroquinolones may be associated with increased risk of abnormal cartilage development; similar effects have not been observed in human studies.96 Use of tetracyclines is hard to justify in pregnancy, since they result in tooth and bone discoloration. Rifamycins are associated with an increased risk of fetal damage and bleeding in both mother and neonate.97 Dornase use has been described in pregnancy,98 but safety data are limited. Safety data on use of pancreatic enzyme replacement therapy are limited,99,100 but use is likely justifiable. Vitamin replacement can be used in pregnancy; however, vitamin A supplementation should not exceed 10,000 U daily because of teratogenic effects. Although sulfonylureas are likely safe in pregnancy, they are associated with an increased risk of fetal hypoglycemia. Insulin is the preferred glucose-lowering drug.
Vaginal delivery is the expected mode of delivery in CF unless other indications for Cesarean section exist. Epidural anesthesia may help with minimizing minute ventilation and oxygen consumption in patients with reduced lung function. Breastfeeding may not be advisable for women with poor nutritional status.
PNEUMONIA IN THE PREGNANT WOMAN
Pneumonia is one of the leading causes of nonobstetric maternal death in the United States.101 The risk of pneumonia is increased in pregnant women with comorbid conditions, such as asthma, anemia, HIV infection, and tobacco or substance abuse.102 Pregnant women may be more susceptible than nonpregnant women to contracting viral infections, and they usually have more severe disease than those who are not pregnant. Immune alterations associated with normal pregnancy may impair the response to infection and increase the risk of certain infections.
Preterm delivery appears to be the most common complication associated with pneumonia. Other potential complications include low birth weight, intrauterine growth restriction, a fetus that is small for gestational age, placental abruption, preeclampsia or eclampsia, and low Apgar scores.103–105 Varicella infection increases morbidity and mortality in pregnancy and is associated with congenital abnormalities and poor fetal outcomes. Congenital varicella syndrome is associated with high mortality in the first 30 days of life and severe impairment related to neurologic, cardiac, gastrointestinal, and developmental delays in survivors.
In the pregnant patient with pneumonia, antibiotic therapy should be initiated empirically while awaiting confirmatory tests that may aid in narrowing the antimicrobial coverage. During influenza season, antivirals (usually oseltamivir) should be started empirically, as well. Extensive use of oseltamivir during the 2009 H1N1 epidemic was thought not to be associated with any adverse fetal effects. Acyclovir or valacyclovir should be used in patients with a suspicion of varicella pneumonia; those suspected of varicella infection should be admitted for observation. Antibiotic use in the pregnant patient with CF has been discussed previously; Table 97-3 provides additional details. Although use of aminoglycosides or vancomycin theoretically may be associated with hearing and renal dysfunction in the fetus, in most circumstances where there is an indication for one or the other agent, its benefit likely outweighs its risk.
In pregnant women with acute respiratory failure from pneumonia or other causes, decisions regarding oxygenation and ventilation should be predicated on the previously discussed physiology. Airway intubation should be anticipated and performed by the most experienced personnel available, using advanced airway devices and readily accessible equipment, given the high risk of intubation failures in pregnant women.
TUBERCULOSIS: CLINICAL COURSE AND MANAGEMENT DURING PREGNANCY
Tuberculosis (TB) is an important infection of pregnant woman. In the United States, a steady overall decline in the incidence of TB has been observed, but a rise in incidence has been seen in the 15-45 year old age group. As a result, an increase in the prevalence of TB among pregnant woman in certain communities, for example, New York City, has been reported.106 In sub-Saharan Africa, TB is the third leading cause of maternal death, following sepsis and hypertensive disorders of pregnancy.107
Pregnancy was originally believed to worsen the course of TB infection, but this has been shown not to be the case. The risks of TB to the mother and unborn child include intrauterine growth restriction, prematurity, and an increased risk for both maternal and fetal mortality. Transmission of TB to the fetus is also well documented108–110 and may occur through hematogenous spread, by contiguous spread from endometrial TB, or via aspiration of TB-infected amniotic fluid. If neonatal TB is suspected, placental and endometrial tissue can be examined at the time of delivery; however, a workup of suspected active maternal TB should never be delayed until delivery. The diagnosis of TB in pregnancy often requires a high level of suspicion, since fatigue and malaise can be confused with symptoms of pregnancy.
Pulmonary TB is the most common form of TB in pregnancy, accounting for up to 85% of all cases. Consequently, a chest radiograph can be essential in making the diagnosis. Tuberculin skin testing (TST) is the gold standard for screening during pregnancy111 studies on use of Quantiferon testing in this population are limited.112,113 Pregnancy may be an excellent opportunity to screen for latent TB, given patients’ frequent contact with healthcare providers and the added motivation for fetal well-being.
Treatment of latent TB infection (LTBI) in pregnancy may be delayed until the postpartum period in pregnant women at low risk for reactivation.114 This recommendation is based on a study showing a higher risk of INH-induced hepatitis in pregnant women compared with non-concurrent controls.115 However, in the study noted, the risk of INH-induced fatal hepatitis in both the active treatment and the control group was quite low. Conversion to active TB infection in patients coinfected with HIV is significant; therefore, immediate treatment should be initiated in coinfected patients to avoid placing the neonate, mother, and community at risk.
Active, untreated TB infection in a pregnant woman is more of a hazard than the treatment. When active TB is confirmed, treatment should not be delayed. Treatment is similar to that in the absence of pregnancy. First-line therapies for TB, including rifampin, INH, and ethambutol, have been shown to be safe in pregnancy and unassociated with human fetal malformations.116,117 INH does carry a risk of acute hepatitis, especially if the patient is treated concomitantly with rifampin. Pyrazinamide is less well studied. Although the World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease note that the use of pyrazinamide is justifiable in pregnancy, the Center for Disease Control (CDC) does not recommend it in pregnancy. When drug resistance is suspected, addition of pyrazinamide may be justified. Second-line agents are considered more toxic than first-line agents, and clinical experience to guide their use and safety is lacking. Streptomycin should be avoided because of its fetal ototoxicity, while amikacin and kanamycin carry known fetal nephrotoxicity. Para-aminosalicylic acid (PAS) has been used with INH in the past; there has been no indication of teratogenicity among babies whose mothers received the drug.112,113,118
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree