Pulmonary Atresia with Ventricular Septal Defect and Major Aortopulmonary Collaterals
V. Mohan Reddy
Frank L. Hanley
Pulmonary atresia with ventricular septal defect (VSD) and major aortopulmonary collaterals is a complex lesion in which great morphologic variability exists regarding the sources of pulmonary blood flow. The anatomy of the true central pulmonary arteries is also highly variable ranging from normal in size to complete absence. Major aortopulmonary collateral arteries (MAPCAs), probably derived embryologically from the splanchnic vascular plexus, are extremely variable in their size, number, course, origin, arborization, and histopathologic makeup. A given segment of the lung may be supplied solely from the true pulmonary arteries, solely from the aortopulmonary collaterals, or from both, sometimes with connections between the two sources occurring at central or peripheral points and at single or multiple sites. In contrast, the intracardiac morphology of this lesion is relatively straightforward, often with a single anteriorly malaligned VSD, well-developed right and left ventricles, and normal atrioventricular and ventriculoarterial connections. Very uncommonly MAPCAs are present in a similar manner in other lesions like single ventricles, double outlet right ventricle, and transposition complexes.
The ultimate goal of surgical therapy in this lesion is to reconstruct completely separated, in-series pulmonary and systemic circulations. The traditional surgical management strategy for achieving this goal is to embark on a staged reconstruction to centralize the multifocal pulmonary blood supply, recruiting as many lung segments as possible, and then close the VSD and provide egress from the right ventricle to the “unifocalized” pulmonary arterial system. In the past, this always has required multiple operations.
The most important physiologic factor signifying a favorable outcome for these patients after complete repair is the postrepair peak right ventricular pressure. This should be as low as possible. The peak right ventricular pressure depends greatly on the number of lung segments that are unifocalized and on the status of the pulmonary microvasculature in those segments. Another important factor is that the reconstruction must achieve unobstructed delivery of blood from the right ventricle to the pulmonary microvasculature. A number of impediments to achieving this ideal outcome exist. Lung segments can be lost for several reasons. The natural history of these MAPCAs often follows a course of progressive stenosis and occlusion, sometimes making the segment of lung supplied by that collateral inaccessible at the time of unifocalization. Even if accessible, a long-standing severe stenosis of the collateral can lead to distal arterial hypoplasia and underdevelopment of preacinar and acinar vessels and alveoli. In addition, iatrogenic occlusion can occur when these collaterals are unifocalized in stages using nonviable conduits, sometimes resulting in loss of these segments. Finally, MAPCAs without obstruction can rapidly lead to pulmonary vascular obstructive disease in their supplied segments. Similarly, staged unifocalization necessitating the use of modified Blalock-Taussig or central shunts may result in pulmonary vascular obstructive disease.
THERAPEUTIC GOALS AND PATIENT SELECTION
It seems logical that the longer the microvasculature of a given lung segment is left to the hemodynamic vagaries of a MAPCA, the more likely it is that it will either develop pulmonary vascular obstructive disease or involute. Only the “perfectly stenosed” MAPCA may allow normal distal development. Furthermore, stenoses in MAPCAs are well known to progress with time, suggesting that even a “perfectly stenosed” vessel is not likely to remain that way. Extending this logic, it seems clear that the sooner these hemodynamic vagaries can be removed, the greater is the likelihood that the largest number of healthy lung segments can be incorporated into the unifocalized pulmonary circuit. The pulmonary microvasculature taken in aggregate is healthiest at birth and declines thereafter. From these arguments, it seems intuitive that one-stage complete unifocalization and repair early in life gives the greatest chance of achieving a healthy and complete pulmonary vascular bed.
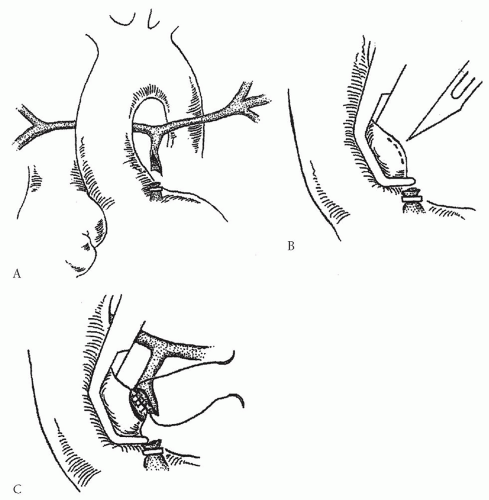
We have prospectively applied a complex surgical management protocol that reflects these goals and principles. The first priority is to completely unifocalize the pulmonary arterial and MAPCA complex via a median sternotomy incision. This removes the MAPCAs from the abnormal physiologic milieu as early as possible. Whenever possible, we also perform intracardiac repair at the first operation. If simultaneous intracardiac repair is not considered advisable because of the concern about unacceptable peak right ventricular pressures, a polytetraflu-oroethylene (PTFE) shunt is constructed between the ascending aorta and the fully reconstructed single-compartment pulmonary arterial system. Based on experience with more than 550 patients, this approach of one-stage complete bilateral unifocalization was performed in about 85% of all patients. In 56%, intracardiac repair was also performed. In 29%, a central shunt was created; intracardiac repair was achieved within 2 years in the great majority of patients in whom shunts were created. The remaining 15% of patients typically fall into two general categories. In one group, centrally confluent true pulmonary arteries were present in association with a relatively complete arborization pattern to most or all lung segments. The source of pulmonary blood flow is via MAPCAs that share dual-supply vascular distribution with the true pulmonary arteries. In this anatomic variant, we construct a neonatal
aortopulmonary window by removing the blind end of the main pulmonary artery from the infundibulum and anastomose it to the ascending aorta, and we concurrently ligate important MAPCAs (Fig. 92.1). If the patients are selected properly, the central source of pulmonary blood flow will distribute to all lung segments. Patients are then evaluated for intracardiac repair at 3 to 6 months of age. In the second group, true pulmonary arteries may or may not be present. The important factor is that the majority of MAPCAs have multiple stenoses at the segmental and subsegmental branches. In our opinion, these patients are best managed by sequential staged thoracotomies at intervals of 3 to 6 months.
aortopulmonary window by removing the blind end of the main pulmonary artery from the infundibulum and anastomose it to the ascending aorta, and we concurrently ligate important MAPCAs (Fig. 92.1). If the patients are selected properly, the central source of pulmonary blood flow will distribute to all lung segments. Patients are then evaluated for intracardiac repair at 3 to 6 months of age. In the second group, true pulmonary arteries may or may not be present. The important factor is that the majority of MAPCAs have multiple stenoses at the segmental and subsegmental branches. In our opinion, these patients are best managed by sequential staged thoracotomies at intervals of 3 to 6 months.
Regardless of which approach is taken, creative surgical techniques are used to achieve native tissue-to-tissue continuity. Allograft patch material is often necessary, but we use it noncircumferentially so that the growth potential of the native tissue is preserved. We limit the use of circumferential nonviable conduits to the central mediastinum.
Ideal age of repair of this lesion based on our extensive experience seems to be between 3 and 6 months in most patients. Our current approach involves the following. If the patient is well balanced physiologically, we prefer to perform this procedure when the patient is between 3 and 6 months of age. However, if the patient is severely cyanotic or has significant pulmonary overcirculation, repair is feasible at an even earlier age. The advantages of early one-stage repair are numerous. Early normalization of cardiovascular physiology and correction of cyanosis is achieved. Protection against pulmonary hypertension, related to high flow either through collaterals or through systemic shunts, is accomplished. The number of operations is reduced. The use of nonviable material in the periphery of the lung is completely eliminated in the great majority of cases. With this approach, the number of patients who can be completely repaired is substantially enhanced.
TECHNIQUE: ONE-STAGE COMPLETE UNIFOCALIZATION AND INTRACARDIAC REPAIR
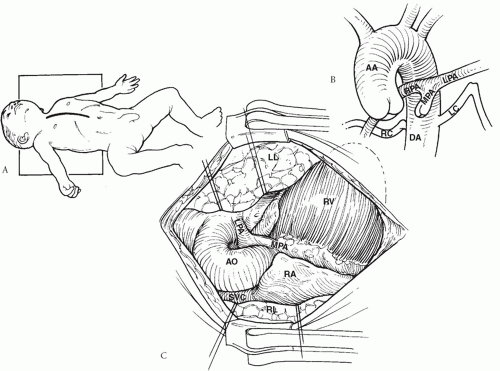
Surgical access to the mediastinum is via a generous midline incision and a median sternotomy (Fig. 92.2). A subtotal thymectomy is performed and the pericardium is harvested and preserved in glutaraldehyde solution. The primary dissection is in the central mediastinum around the trachebronchila tree, subcarinal space,
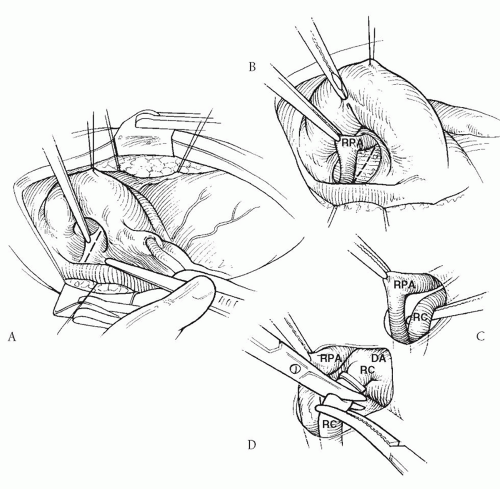
and the space between the superior vena cava and the aorta. The native pulmonary arteries, if present, are dissected out (Fig. 92.3A). Most collaterals from the upper descending aorta can be identified and dissected in the subcarinal space (between the tracheobronchial angle and the roof of the left atrium) by an approach between the right superior vena cava and the aorta (Fig. 92.3B). The floor of the pericardial reflection in the transverse sinus is opened, and the posterior mediastinal soft tissues are dissected using cautery. The descending aorta is then exposed in the posterior mediastinum, and all the collaterals from it are identified, dissected, mobilized, and controlled. The subcarinal approach is an important maneuver not only to gain access to collaterals, which typically arise from this location, but also to provide the most direct avenue for collateral rerouting for direct tissue-to-tissue anastomosis, which would otherwise be impossible. In addition, in some cases collaterals arising from the aortic arch or the neck vessels are exposed and dissected in the superior mediastinum along the trachea or through the pleural cavities. Avenues for collateral rerouting are also developed by opening the pleura on both sides posterior to the phrenic nerves in the hilar regions (Fig. 92.4). If the collaterals need only to be ligated and not unifocalized, then the mediastinal dissection can be minimized by the pleural approach. In such cases, the right pleura is widely opened anterior to the phrenic nerve, the right lung is lifted out of the pleural cavity, and the right-sided collaterals are identified and dissected. Similarly, the left pleura is opened and the left-sided collaterals are identified and dissected. The pleural approach is also useful to quickly gain control of all the collaterals in unstable patients before or immediately after cardiopulmonary bypass is instituted.
and the space between the superior vena cava and the aorta. The native pulmonary arteries, if present, are dissected out (Fig. 92.3A). Most collaterals from the upper descending aorta can be identified and dissected in the subcarinal space (between the tracheobronchial angle and the roof of the left atrium) by an approach between the right superior vena cava and the aorta (Fig. 92.3B). The floor of the pericardial reflection in the transverse sinus is opened, and the posterior mediastinal soft tissues are dissected using cautery. The descending aorta is then exposed in the posterior mediastinum, and all the collaterals from it are identified, dissected, mobilized, and controlled. The subcarinal approach is an important maneuver not only to gain access to collaterals, which typically arise from this location, but also to provide the most direct avenue for collateral rerouting for direct tissue-to-tissue anastomosis, which would otherwise be impossible. In addition, in some cases collaterals arising from the aortic arch or the neck vessels are exposed and dissected in the superior mediastinum along the trachea or through the pleural cavities. Avenues for collateral rerouting are also developed by opening the pleura on both sides posterior to the phrenic nerves in the hilar regions (Fig. 92.4). If the collaterals need only to be ligated and not unifocalized, then the mediastinal dissection can be minimized by the pleural approach. In such cases, the right pleura is widely opened anterior to the phrenic nerve, the right lung is lifted out of the pleural cavity, and the right-sided collaterals are identified and dissected. Similarly, the left pleura is opened and the left-sided collaterals are identified and dissected. The pleural approach is also useful to quickly gain control of all the collaterals in unstable patients before or immediately after cardiopulmonary bypass is instituted.
In stable patients, as many collaterals as possible are permanently ligated at their origin, mobilized, and unifocalized without cardiopulmonary bypass (Fig. 92.3C and 92.3D). When the patient’s oxygenation reaches a compromising level, cardiopulmonary bypass is instituted, and the remainder of the collaterals is unifocalized at mild-to-moderate hypothermia with the heart beating. A calciumsupplemented blood prime is used in the cardiopulmonary bypass pump circuit to maintain normal cardiac function. During
the unifocalization process, the emphasis is on avoiding synthetic or allograft conduits in the periphery and on achieving unifocalization by native tissue-to-tissue anastomosis. One or more of the following techniques of unifocalization are generally used in these patients:
the unifocalization process, the emphasis is on avoiding synthetic or allograft conduits in the periphery and on achieving unifocalization by native tissue-to-tissue anastomosis. One or more of the following techniques of unifocalization are generally used in these patients:
Side-to-side anastomosis of the collateral to the central pulmonary arteries, thereby augmenting the hypoplastic central pulmonary arteries
Side-to-side anastomosis of collateral to collateral or of collateral to peripheral native pulmonary artery
End-to-side anastomosis of collateral to collateral or of collateral to native pulmonary artery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree