The association between uric acid and cardiovascular disease is incompletely understood. In particular, the prognostic value of uric acid in patients with acute coronary syndromes who undergo percutaneous coronary intervention has not been studied. This study included 5,124 patients with acute coronary syndromes who underwent percutaneous coronary intervention: 1,629 with acute ST-segment elevation myocardial infarction, 1,332 with acute non–ST-segment elevation myocardial infarction, and 2,163 with unstable angina. The primary end point was 1-year mortality. Patients were divided into quartiles according to uric acid level as follows: quartile 1, 1.3 to <5.3 mg/dl; quartile 2, 5.3 to <6.3 mg/dl; quartile 3, 6.3 to <7.5 mg/dl; and quartile 4, 7.5 to 18.4 mg/dl. There were 450 deaths during follow-up: 80 deaths in quartile 1, 77deaths in quartile 2, 72 deaths in quartile 3, and 221 deaths in quartile 4 of uric acid (Kaplan-Meier estimates of 1-year mortality 6.4%, 6.2%, 5.6%, and 17.4%, respectively; unadjusted hazard ratio 3.05, 95% confidence interval 2.54 to 3.67, p <0.001 for fourth vs first quartile of uric acid). After adjustment for traditional cardiovascular risk factors, renal function, and inflammatory status, the association between uric acid and mortality remained significant, with a 12% increase in the adjusted risk for 1-year mortality for every 1 mg/dl increase in the uric acid level. Uric acid improved the discriminatory power of the predictive model regarding 1-year mortality (absolute integrated discrimination improvement 0.008, p = 0.005). In conclusion, elevated levels of uric acid are an independent predictor of 1-year mortality across the whole spectrum of patients with acute coronary syndromes treated with percutaneous coronary intervention.
Acute coronary syndromes (ACS) remain a leading cause of morbidity and mortality worldwide. Despite the application of various risk stratification schemes, ACS remain poorly characterized in terms of risk prediction. Uric acid is the end product of purine catabolism in humans and is readily tested in routine clinical practice. Although the possibility of an association between elevated uric acid level and cardiovascular disease has been recognized for >130 years, the role of acid uric as a risk factor or a risk marker for cardiovascular disease remains a debatable issue. The association between elevated uric acid and increased risk for mortality has been investigated in the general population, and in patients with congestive heart failure, hypertension, diabetes, angiographically confirmed coronary artery disease, and acute myocardial infarction. No studies have addressed the prognostic value of this biomarker across the whole spectrum of patients with ACS. Thus, the aim of this study was to investigate whether there is an association between acid uric level and mortality across the whole spectrum of patients with ACS who undergo percutaneous coronary intervention (PCI).
Methods
This study included 5,124 patients with ACS who underwent PCI at the German Heart Center in Munich from March 2000 to December 2009. Of these, 1,629 patients had acute ST-segment elevation myocardial infarction (STEMI), 1,332 had acute non–ST-segment elevation acute myocardial infarction (NSTEMI), and 2,163 had unstable angina. The diagnosis of STEMI was based on the presence of chest pain lasting ≥20 minutes associated with typical changes on surface electrocardiography (ST-segment elevation ≥0.1 mV in ≥2 limb leads or ≥0.2 mV in ≥2 contiguous precordial leads or complete left bundle branch block of new onset). Unstable angina was diagnosed using Braunwald’s criteria plus documentation of significant coronary artery disease on coronary angiography. Diagnosis of NSTEMI required clinical and electrocardiographic criteria similar to those of unstable angina plus elevated troponin T (>0.03 μg/L) or creatine kinase or creatine kinase-myocardial band. In all included patients, the diagnosis of ACS was confirmed by coronary angiography. Weight and height were measured with patients wearing light clothing without shoes, and body mass index was calculated. The estimated glomerular filtration rate was calculated using the Cockcroft-Gault formula. Detailed criteria of the diagnosis of cardiovascular risk factors are given in another publication from our group. Patients with acute inflammatory states, known malignancies, or advanced kidney disease were not included in the present study. Written informed consent was obtained from all patients. The study was carried out in accordance with the Declaration of Helsinki and was approved by the institutional ethics committee.
Angiographic data were analyzed in the quantitative angiographic core laboratory. Coronary artery disease was confirmed by the presence of coronary stenoses ≥50% luminal obstruction in ≥1 of the 3 main coronary arteries. Offline analysis of digital angiograms was performed in the core laboratory using an automated edge detection system (CMS; Medis Medical Imaging Systems, Leiden, The Netherlands). The global left ventricular ejection fraction was determined by left ventricular angiography using the area-length method. The diagnosis of stroke required confirmation by computed tomography or magnetic resonance imaging of the head. Stent implantation and periprocedural care were performed according to standard criteria. Postinterventional antiplatelet therapy consisted of clopidogrel (300 or 600 mg as a loading dose followed by 75 mg/day for ≥4 weeks to 6 months) and aspirin (200 mg/day administered orally and continued indefinitely). Drug-eluting stents were implanted in 3,366 patients (66%).
Blood samples were obtained before angiography in all patients. The uric acid concentration in plasma was determined with an enzymatic colorimetric test on a Cobas Integra 800 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Uricase cleaves uric acid to form allantoin and hydrogen peroxide, which reacts to form a quinoneimine dye. The measuring range in plasma is 0.20 to 25 mg/dl (11.9 to 1,500 μmol/L). The lower detection limit of the test is 0.20 mg/dl (11.9 μmol/l). The reference range for men is 3.4 to 7.0 mg/dl (202.3 to 416.5 μmol/L) and for women is 2.4 to 5.7 mg/dl (142.8 to 339.2 μmol/L).
The primary end point of the study was all-cause mortality. Nonfatal myocardial infarction, stroke, and target lesion revascularization were also evaluated. The follow-up protocol after discharge consisted of a phone interview at 1 month after the procedure, a visit at 6 months, and a phone interview at 12 months. Information about death was obtained from hospital records, death certificates, or phone contact with relatives of the patient or the referring physician. Patients who had cardiac symptoms underwent complete clinical, electrocardiographic, and laboratory evaluations. Follow-up information was obtained and adjudication of adverse events was performed by medical staff members unaware of clinical diagnosis, therapy received, or uric acid levels.
The normality of data distribution was assessed using a 1-sample Kolmogorov-Smirnov test. Data are presented as medians with 25th and 75th percentiles or as counts and proportions (percentages). Categorical data were compared using chi-square test. Continuous data were compared using Kruskal-Wallis rank-sum tests. A multiple linear regression model was used to identify the independent correlates of uric acid level. All variables listed in Table 1 were entered into the model. Survival analysis was performed by applying the Kaplan-Meier method. Univariate and multivariate Cox proportional-hazards models were used to assess the association between uric acid (entered into the model as a continuous variable) and 1-year mortality. All variables listed in Table 1 and the interaction between gender and uric acid level were entered into the model. The discriminatory power of the model regarding mortality with and without the inclusion of uric acid was assessed by calculating the C statistic in the Cox proportional-hazards model and the integrated discrimination improvement according to Pencina et al. All analyses were performed using S-plus (Insightful Corporation, Seattle, Washington). Two-tailed p values <0.05 were considered to indicate statistical significance.
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Value |
|---|---|---|---|---|---|
| (n = 1,271) | (n = 1,261) | (n = 1,300) | (n = 1,292) | ||
| Age (years) | 67.7 (59.2, 76.4) | 67.0 (58.8, 74.5) | 68.1 (59.1, 75.2) | 69.7 (61.2, 77.5) | <0.001 |
| Women | 546 (43.0%) | 293 (23.2%) | 250 (19.2%) | 273 (21.1%) | <0.001 |
| Body mass index (kg/m 2 ) | 25.7 (23.4, 28.1) | 26.3 (24.5, 29.0) | 27.4 (25.0, 29.8) | 27.7 (25.0, 30.5) | <0.001 |
| Diabetes mellitus | 352 (27.7%) | 303 (24.0%) | 346 (26.6%) | 430 (33.3%) | <0.001 |
| Arterial hypertension | 785 (61.8%) | 802 (63.6%) | 826 (63.5%) | 742 (57.4%) | 0.003 |
| Current smoker | 295 (23.2%) | 281 (22.3%) | 272 (20.9%) | 235 (18.2%) | 0.011 |
| Hypercholesterolemia (≥240 mg/dl) | 758 (59.6%) | 817 (64.8%) | 816 (62.8%) | 810 (62.7%) | 0.062 |
| Previous myocardial infarction | 268 (21.1%) | 284 (22.5%) | 344 (26.5%) | 394 (30.5%) | <0.001 |
| Previous coronary artery bypass surgery | 156 (12.3%) | 156 (12.4%) | 171 (13.2%) | 189 (14.6%) | 0.261 |
| Clinical presentation | <0.001 | ||||
| Unstable angina | 525 (41.3%) | 550 (43.6%) | 604 (56.5%) | 484 (37.5%) | |
| NSTEMI | 281 (22.1%) | 323 (25.6%) | 299 (23.0%) | 429 (33.2%) | |
| STEMI | 465 (36.6%) | 388 (30.8%) | 397 (30.5%) | 379 (29.3%) | |
| Cardiogenic shock | 38 (3.0%) | 42 (3.3%) | 51 (3.9%) | 146 (11.3%) | <0.001 |
| Number of coronary artery narrowed | <0.001 | ||||
| 1 | 310 (24.4%) | 272 (21.5%) | 258 (19.8%) | 197 (15.2%) | |
| 2 | 342 (26.9%) | 340 (27.0%) | 360 (27.7%) | 314 (24.3%) | |
| 3 | 619 (48.7%) | 649 (51.5%) | 682 (52.5%) | 781 (60.4%) | |
| Multivessel disease | 961 (75.6%) | 989 (78.5%) | 1,042 (80.2%) | 1,095 (84.8%) | <0.001 |
| Left ventricular ejection fraction (%) | 54.0 (45.0, 61.0) | 55.0 (45.0, 61.0) | 54.0 (44.0, 61.0) | 49.0 (37.0, 58.0) | <0.001 |
| Uric acid (mg/dl) | 4.6 (4.0, 5.0) | 5.8 (5.5, 6.0) | 6.8 (6.5, 7.1) | 8.5 (7.9, 9.5) | <0.001 |
| Serum creatinine (mg/dl) | 0.9 (0.7, 1.0) | 0.9 (0.8, 1.1) | 1.0 (0.9, 1.3) | 1.2 (1.0, 1.5) | <0.001 |
| Glomerular filtration rate (ml/min) | 79.2 (61.1, 100.4) | 82.6 (63.4, 105.1) | 79.8 (59.3, 102.0) | 63.2 (44.4, 88.1) | <0.001 |
| C-reactive protein (mg/L) | 3.4 (1.2, 11.7) | 3.2 (1.2, 9.6) | 3.6 (1.4, 11.2) | 5.8 (1.9, 17.7) | <0.001 |
Results
A total of 5,124 patients with ACS were included in the study. Quartiles of uric acid concentrations were as follows: quartile 1, 1.3 to <5.3 mg/dl; quartile 2, 5.3 to <6.3 mg/dl; quartile 3, 6.3 to <7.5 mg/dl; and quartile 4, 7.5 to 18.4 mg/dl.
Table 1 lists baseline characteristics according to quartiles of uric acid. Patients with uric acid levels in the upper quartile were older, were less often women, and had higher body mass indexes. The upper quartile included higher proportions of those with previous myocardial infarction, diabetes, and multivessel disease and lower proportions of those with arterial hypertension and current smoking. These patients presented more often with cardiogenic shock, had lower left ventricular ejection fractions, had lower glomerular filtration rates, and had higher creatinine and C-reactive protein levels. There were also differences among patients of various uric acid quartiles with regard to clinical presentation ( Table 1 ). Only 137 patients (2.7%) were receiving allopurinol on admission, with no difference among the groups (p = 0.11).
Independent determinants of uric acid level were identified using multivariate linear regression (see “Methods” for the variables entered into the model). The following variables were identified by the model as independently associated with elevated levels of uric acid: elderly age, male gender, higher body mass index, absence of diabetes, clinical presentation, cardiogenic shock, lack of previous coronary artery bypass surgery, left ventricular ejection fraction, and glomerular filtration rate ( Table 2 ).
| Variable | β | p Value |
|---|---|---|
| Age | 0.025 | <0.001 |
| Female gender | −0.974 | 0.001 |
| Body mass index | 0.138 | <0.001 |
| Diabetes mellitus | −0.115 | 0.035 |
| Clinical presentation (STEMI vs angina) | −0.200 | <0.001 |
| Cardiogenic shock | 0.655 | <0.001 |
| Previous coronary artery bypass surgery | −0.178 | 0.020 |
| Left ventricular ejection fraction | −0.016 | <0.001 |
| Glomerular filtration rate | −0.025 | <0.001 |
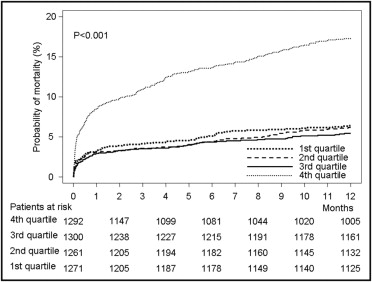
There were 450 deaths within the first year of follow-up. Deaths according to quartile of uric acid were as follows: 80 deaths in quartile 1, 77 in quartile 2, 72 in quartile 3, and 221 in quartile 4 (Kaplan-Meier estimates of 1-year mortality 6.4%, 6.2%, 5.6%, and 17.4%, respectively; unadjusted hazard ratio [HR] 3.05, 95% confidence interval [CI] 2.54 to 3.67, p <0.001 for fourth quartile vs first quartile; Figure 1 ). The other univariate associates of 1-year mortality are listed in Table 3 .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


