Chapter 16 Prognostic Implications of MPI Stress SPECT
INTRODUCTION
At a time when other modalities—specifically cardiac computed tomography (CT), cardiac magnetic resonance (CMR), and positron emission tomography (PET)—are increasingly being used in the assessment and management of patients with known or suspected coronary artery disease (CAD),1 the question of why stress myocardial perfusion single-photon emission computed tomography (SPECT), or MPS, continues to be the most commonly utilized of these modalities must be asked. Cardiac CT and CMR have superior resolution, hence can successfully image coronary arteries, left ventricular size, shape, and wall thickness, as well as valves, pericardium, and other clinically relevant structures. Further, coronary CT angiography (CCTA) has the promise of imaging atherosclerotic plaque burden, morphology, and composition. In this era of newer, advanced modalities, will MPS still have a home?
PRINCIPLES OF RISK STRATIFICATION: PATIENT SELECTION AND METRICS OF RISK
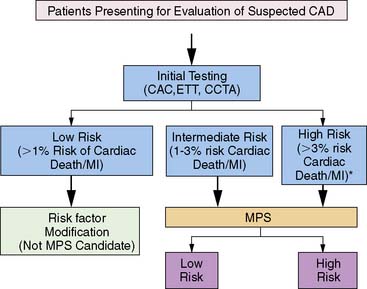
Several concepts define the basis of risk stratification after stress imaging; generally speaking, these principals hold true for all testing modalities. First, with respect to appropriate selection of patients for testing, the basic concept underlying the use of nuclear testing for risk stratification is that only those patients who can be successfully further stratified (or restratified) in a cost-effective manner would be appropriate patients for stress MPS.2 While MPS has been shown to successfully risk stratify multiple, diverse populations,3 it is cost-effective only when applied to intermediate and high-risk patients,3–6 and its use should be limited to these populations.7
Practically, optimal risk stratification is based on the hypothesis that the risk associated with a normal stress imaging study is sufficiently low that aggressive CAD management and therapeutics will not further improve patient outcomes.3,8,9 Hence, for example, it is commonly held that invasive coronary angiography and coronary interventions are less frequently performed in patients with normal stress imaging studies than in symptomatic patients in whom these studies are not performed.6
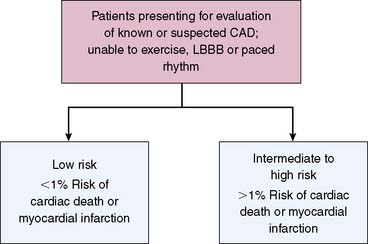
On the other hand, patients with abnormal stress imaging results are at greater risk of adverse events, thus resulting in risk stratification in its most basic form relative to normal MPS results (Fig. 16-1). Also, these patients are potential candidates for intervention,10–12 and the magnitude of their risk is related to the extent and severity of the imaging abnormalities. Based on this premise, outcomes data from an imaging modality should initially be examined for two patterns: (1) risk of adverse events after a normal study and (2) relationship between risk and increasing test abnormality.2
To date, examination of MPS test performance has focused on significant clinical events as endpoints—all cause death, cardiac death, and nonfatal myocardial infarction. In this chapter, we will focus on studies evaluating the association of MPS with major events. For purposes of risk assessment, it has been proposed that low risk be defined as a less than 1% annual cardiac mortality rate, intermediate risk defined by the range of 1% to 3% per year, and high risk as greater than 3% per year.13 It is likely that in the future, studies will increasingly use endpoints related to resource utilization (hospitalization of cardiac causes, emergency department visits, referral to downstream interventions and procedures) to better capture the association of MPS with the global cardiac outcomes, thus better defining its role in patient care.2
The use of metrics and thresholds to define the success of risk stratification by testing is challenging. Historically in nuclear cardiology, risk stratification was considered successful if patients with normal scans had either a hard event rate or a mortality rate (varying with publication) of less than 1% per year of follow-up, while patients with abnormal scans have rates exceeding 1%. As will be discussed, the use of thresholding to define levels of risk is problematic. The effectiveness of stratification may also be judged by the ratio of risk in patients with abnormal, compared to those with normal, scans (as measured by a relative risk or odds ratio); increasing relative risks indicate increasing effectiveness in risk stratification.2
Risk of Adverse Events After a Normal Imaging Study
To date, there is extensive literature that supports the concept that a normal stress SPECT study is associated with a low risk of hard events (cardiac death or nonfatal myocardial infarction). A pooled analysis from 19 series in the literature comprising 39,173 patients with normal stress SPECT studies, followed for an average of 2.3 years, showed an annual death or myocardial infarction rate of 0.6%.8 Further, an American Society of Nuclear Cardiology position statement on normal SPECT results reported the very low likelihood (<1%) of adverse events such as cardiac death or myocardial infarction for at least 12 months, independent of gender, age, symptom status, past history of CAD, presence of anatomic CAD, imaging technique, or isotope.14
A closer scrutiny of the published literature reveals inconsistency in the message of the statements in the previous paragraph. In general, these studies have suggested that this low risk is independent of imaging type (SPECT versus planar), the type of stress performed (exercise versus pharmacologic), the radiopharmaceutical used, patients’ clinical characteristics, patients’ prior history of CAD, the results of stress testing, as well as many other factors. However, studies in patients undergoing pharmacologic stress, a population at higher risk and with more comorbidities than patients undergoing exercise stress, have reported hard event rates of 1.3% to 2.7% per year, suggesting that underlying clinical risk and previous CAD may influence event rates after a normal MPS.15–21
These studies encompass cohorts undergoing dipyridamole stress,15 patients aged 70 years or older,22 [Hachamovitch, 2003 #11] patients with stable chest pain undergoing dipyridamole stress,18 patients with diabetes mellitus undergoing adenosine stress,19,21,19–21 and patients undergoing dobutamine stress.17 This paradigm is particularly challenged with diabetic patients. Diabetic patients have been found to have strikingly higher event rates after normal MPS,23 with a number of studies reporting annual hard event rates of 2.0% or more. Interestingly, the event rates in diabetics after stress echocardiography are even greater.24
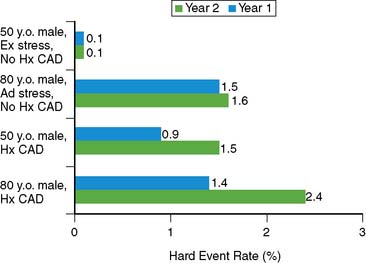
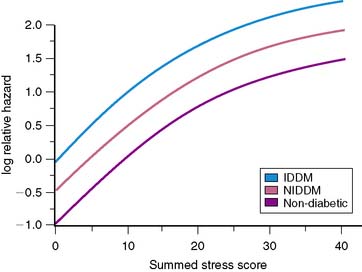
The issue of variability in risk after a normal MPS and the temporal characteristics of this risk (e.g., its “warranty” period) was addressed by a series of 7376 patients with normal stress MPS.21 This study identified a number of variables: the use of pharmacologic stress, the presence of known CAD, diabetes mellitus (in particular, female diabetics), and advanced age as markers of increased risk and shortened time to risk (e.g., risk in the first year of follow-up was less than in the second year). This study attributed the increased risk after normal MPS in a small subset of patients to the presence of comorbidities that increased the baseline risk of these patients (diabetes mellitus, age, inability to exercise, previous CAD). The more of these characteristics present, the greater the risk after a normal MPS test (Figs. 16-2 and 16-3).
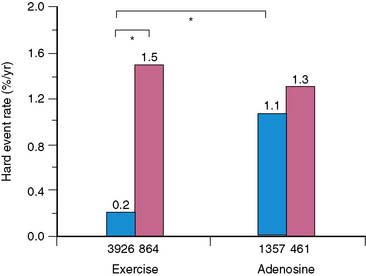
Figure 16-2 Hard event rates (% per year) in patients without history of known coronary artery disease (blue bars) versus with history of known coronary artery disease (pink bars) undergoing exercise (left) or adenosine (right) stress. Numbers under bars represent number of patients within category.21 *P < 0.001.
A review by Kalamesh et al.24 addresses the issue of event rates exceeding the threshold of 1% risk per year in specific patient subsets and posed the question of whether it is a failure of the test or a characteristic of the patient. If it is a failure of the test, the implication would be that the test should not be relied on in certain patient subsets (e.g., diabetic patients with suspected CAD should not go to MPS). If the higher event rates after a normal study result from a characteristic of the patient, then it becomes important to set aside generalized thresholds and define what patient-specific event rates are acceptable after a normal study. An alternative answer is that the failure is of the paradigm of defining risk by a single threshold. Given the diversity of patients presenting for evaluation for risk of cardiac events and their wide pretest range of risk of adverse events, the definition of low risk after testing (i.e., the posttest risk) needs to take into account the patient’s pretest risk as well as the characteristics of the test, similar to the methods applied to calculate the pretest and posttest likelihood of CAD. Thus, although normal MPS results are associated with low absolute risk in most patient cohorts, care must be taken in assessing post-MPS risk in patients with comorbidities and risk factors.
Relationship Between Risk and the Extent and Severity of Imaging Results
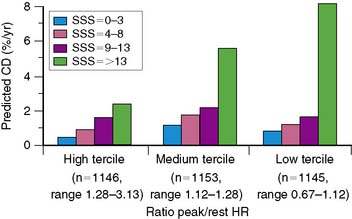
In general, it is safe to say that a close relationship exists between the extent and severity of perfusion abnormalities on stress MPS and subsequent risk of adverse outcomes (Fig. 16-4).11,12,25 Several characteristics of abnormal studies are worth highlighting. First, even after first stratifying a cohort by their pre-MPS risk, MPS results will still achieve further risk stratification in all levels of pre-MPS risk (Figs. 16-5 and 16-6).2,12 This pattern of results can be considered to be a demonstration of clinical incremental prognostic value.12 In a similar relationship, nonperfusion SPECT imaging variables such as transient ischemic dilation of the left ventricle and variables reflecting regional and global LV function add useful prognostic information to sole assessment of extent and severity of perfusion defects.3 Further, in patients undergoing vasodilator stress, the presence of a lower baseline heart rate and a greater peak heart rate were both associated with decreased risk (Fig. 16-7).26
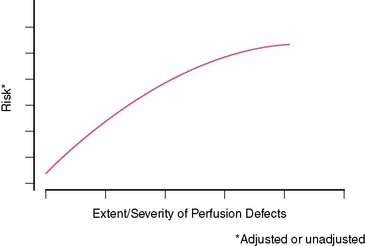
Figure 16-4 A generalized schematic representing the relationship between the extent and severity of MPS defects and post-MPS risk. The shape of this curve (flattening of the curve at high levels of defect extent and severity) is related to the use of revascularization in higher-risk patients, thus reducing the observed risk in MPS populations.2,5 This curve is shifted up or down (change in risk for any MPS result) by the patients’ baseline (pre-MPS) risk.
(From Hachamovitch R, Di Carli MF: Contemporary reviews in cardiovascular medicine: Methods and limitations of assessing new noninvasive tests II. Outcomes-based validation and reliability assessment of noninvasive testing. Circulation 117:2793-2801, 2008.)
Added Value of Gated SPECT
Since gated SPECT has become routine only recently, there are several reports of its incremental value over perfusion in assessing prognosis. The first report showed that poststress LVEF and LV end-systolic volume (ESV), as measured by gated SPECT, provided incremental information over the perfusion defect assessment in the prediction of cardiac death.27 The results of these studies were in large part confirmed by reports from other centers.28,29
USE OF MPS IN SPECIFIC PATIENT POPULATIONS
A principal strength of nuclear cardiology is that large databases have been accumulated, resulting in evidence documenting the effectiveness of MPS for risk stratification of appropriately selected patients comprising the full spectrum of patients with suspected or chronic CAD. This evidence has resulted in many class I indications for the use of stress MPS.8 Several specific lines of evidence are described in the following sections.
Patient Cohorts Defined by CAD Likelihood and ECG Criteria
Patients With an Intermediate Likelihood of CAD or Indeterminate Treadmill Test
A number of studies support a role for MPS for risk stratification in patients with either intermediate post-ETT likelihood of CAD or patients with uninterpretable ETT results.8 An initial report from Cedars-Sinai demonstrated that MPS was effective in risk stratification and driving management of patients with an intermediate Duke Treadmill Score (DTS).12 Subsequent studies revealed that the cost-effectiveness of a strategy utilizing MPS is cost saving versus a strategy of direct referral to catheterization in these patients.4,30 Similar results were shown in subsequent multicenter studies reporting event rates and catheterization rates.8
Patients With Normal Resting ECG Able to Exercise
Patients with normal resting electrocardiograms (ECGs) have been a problematic group with respect to their appropriateness for stress imaging. On the one hand, in clinical practice, these patients represent a large subgroup regularly referred to MPS when, taking into account various clinical factors, the post-ETT risk is not low. On the other hand, patients with a normal resting ECG in general are likely (92% to 96%) to have normal LV function31,32 and to have an excellent prognosis.4,33 The reticence of many writers of guidelines to embrace the use of MPS in these patients is based in part on a study from the Mayo Clinic.34 Although these investigators demonstrated that MPS was able to reclassify the likelihood of anatomically severe CAD after considering clinical and ETT data, so few patients were reclassified with respect to their likelihood that MPS was not cost-effective. Hence, previous guidelines did not recommend the use of MPS in these patients,13 and use of MPS is controversial.
More recently, however, a study designed to parallel the study mentioned was reported, with the important distinction that it employed a prognostic rather than anatomic endpoint.4 Contrary to findings of the prior study using the anatomic definition of high risk, this study reported that selective use of MPS in patients with intermediate to high post-ETT CAD likelihood yielded significant risk stratification, statistical incremental value, and cost-effectiveness in predicting hard events (Fig. 16-8). A subsequent report has shown that patients with a high clinical risk (based on a clinical score combining age, sex, prior MI, and diabetic state) are at too high pretest risk to be classified as low risk by nonimaging exercise testing alone. The authors suggested that initial stress MPS testing might be appropriate in this group.35
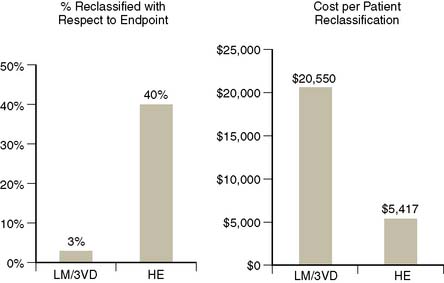
Figure 16-8 Comparison of two studies examining clinical and cost-effectiveness of MPS in patients with normal rest ECG and no prior history of CAD, one using an anatomic endpoint (presence of left main or three vessel CAD; 411 patients)34 and a second a prognostic endpoint (hard events; 3058 patients with a 1.6-year follow-up).4 On the left, the percent of patients reclassified with respect to their likelihood of the endpoint; on the right, the cost per patient reclassified. In the first study, very few patients were reclassified with respect to their risk of severe anatomic CAD, thus the cost per reclassification was unacceptably high. On the other hand, the use of a prognostic endpoint in this population resulted in considerably more patients reclassified with respect to the endpoint, thus a much lower cost per reclassification.
Thus, while referral of functionally capable patients with a normal ECG to MPS is considered inappropriate according to the ACC appropriateness criteria,7 recent studies indicate that there are patient groups able to exercise with normal rest ECG (such as those with a high pretest likelihood of CAD5 and the elderly) in which MPS may be indicated.
Patients With Normal Resting ECG Unable to Exercise
In patients unable to exercise to a target heart rate, there is a clear consensus supporting MPS using pharmacologic stress as the initial test in symptomatic male and female patients with intermediate or high pretest likelihood of CAD.8,36 As shown in multiple previous studies, the inability to exercise per se is itself an incremental predictor of adverse outcomes8,11,21,37 on par with prior CAD, abnormal MPS, or other high-risk markers. Despite the higher event rates for any test result, for patients who have a normal resting ECG and an intermediate to high likelihood of CAD but are unable to exercise, vasodilator stress MPS has been shown to be effective for both CAD diagnosis and risk stratification.8,20,38 The relative effectiveness of risk identification tends to be superior with pharmacologic versus exercise stress (due to the considerably greater event rates in the setting of abnormal MPS with pharmacologic stress).
Patients With High Pretest Likelihood of CAD
Historically, symptomatic patients without known CAD who have a high likelihood of CAD based on age, sex, symptoms, and risk factors were considered candidates for direct referral to revascularization. This was based on the argument that a normal ETT or MPS result would not be sufficient to reclassify the patient as having a low likelihood of CAD, hence precluding the ability for the clinician to confidently exclude the presence of angiographically significant CAD, resulting in diagnostic uncertainty. However, prognostically, it might be possible to classify such patients as low risk. A study assessing the clinical and cost-effectiveness of MPS in 1270 patients with a high CAD likelihood (=0.85) revealed that the majority of these patients had a normal MPS study (which had an associated hard event rate of 1.3%).5 A strategy incorporating initial testing with MPS in these patients to guide decision for coronary angiography was shown to be cost-effective.5,6 The ACC appropriateness criteria support MPS in high-likelihood patients who have an interpretable or uninterpretable ECG, as well as for those able or unable to exercise.7
Patients With Left Bundle Branch Block (See Chapter 5)
At the current time, the guidelines support the use of MPS in symptomatic patients with left bundle branch block, since ETT is not an option in these patients,8 and the rate of false-positive perfusion defects is observed less frequently with vasodilator stress. This is further supported by the finding of a greater specificity associated with vasodilator stress compared to exercise stress in these patients with similar sensitivities.39 This approach has also been found to be prognostically valuable and predictive of adverse outcomes in LBBB patients.40,41 Whereas patients with LBBB and normal MPS have relatively low event rates, patients with LBBB and abnormal MPS results tend to have greater event rates for any defect size compared to other patients.
Patients With LVH or Atrial Fibrillation
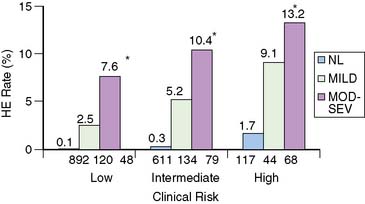
In patients with LVH, exertional ST-segment depression is frequently associated without significant CAD. MPS has been shown to be similarly effective in patients with and without LVH for identifying obstructive disease and for risk stratification. In one report, patients with LVH and a low-risk MPS had a less than 1% annual risk of cardiac death or nonfatal myocardial infarction, while the annual cardiac death or nonfatal myocardial infarction rates ranged from 4.9% for mildly abnormal scans to 10.3% for those with moderate to severely abnormal MPS.42
In asymptomatic patients with new-onset atrial fibrillation, the use of stress MPS in patients with a high pretest risk is considered appropriate7 in view of a higher baseline clinical risk, resulting in higher expected cardiac events. A study on the prognostic value of MPS in patients with atrial fibrillation reported an annualized cardiac death rate of 1.6% in the setting of a normal MPS result versus 0.4% for a normal MPS in patients without AF (P < 0.001).43 These authors also reported that a mildly abnormal MPS study in patients with atrial fibrillation is associated with a higher risk than in those without atrial fibrillation, potentially implying the need for a different threshold for determining the appropriateness of referral of these patients catheterization.
Patient Cohorts Defined by Risk Factors and Demographics
Asymptomatic Patients
The diagnostic and prognostic value of stress MPS in asymptomatic populations has been previously examined. The routine use of any test for detection of CAD in a population at low risk/low prevalence of CAD is unlikely to be effective and will be associated with high cost-effectiveness ratios and low positive predictive values. Nonetheless, these evaluations are often performed in patients with high-risk occupations (e.g., pilots, firefighters).8 However, specific asymptomatic populations who are at intermediate to high risk will be candidates for MPS. For example, asymptomatic siblings of patients with manifest CAD have been found to be at elevated risk of developing CAD and at higher risk of adverse outcomes subsequently.44 Similarly, certain diabetic patients and women, the former often asymptomatic and the latter with atypical or noncardiac symptoms, also fall into the asymptomatic category but may well be MPS candidates, depending on their estimated risk. The ACCF/ASNC appropriateness criteria consider the use of MPS in asymptomatic patients with a high Framingham risk and those classified as CAD risk equivalent (diabetics) to be appropriate.7
Nuclear Imaging in Patients With Diabetes Mellitus
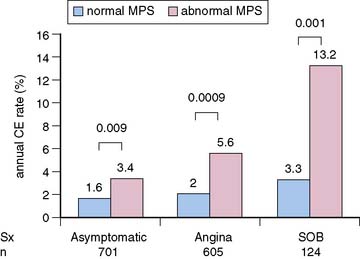
Multiple reports, to date, have supported the value of MPS for risk stratification of diabetic patients.3 Event rates associated with any MPS result are greater in diabetic compared to nondiabetic patients (Fig. 16-9).19,20,45 These findings were confirmed in a multicenter series.46 In the latter study, diabetic women had the worst outcome for any given extent of myocardial infarction. In patients with normal MPS results, survival worsened sooner in diabetic compared to nondiabetic patients, suggesting that retesting of diabetics with normal studies might be needed earlier than in nondiabetics.21 In a study of 1430 diabetic patients (701 asymptomatic) followed for a mean of 2.1 years after MPS, Zellweger et al.47 reported that significant risk stratification was seen when comparing normal and abnormal MPS results in asymptomatic diabetic patients. They observed 1.6% and 3.4% annual hard event rates in those with normal and abnormal MPS scans, respectively. Of interest, this same report revealed that while risk stratification was also observed in the diabetics with angina and shortness of breath, the event rates for both the normal and abnormal scan groups were higher in these groups than in the asymptomatic diabetics (Fig. 16-10). It has been shown that 22% of asymptomatic diabetic patients have ischemia by adenosine MPS,48 but the preponderance of these patients with abnormal MPS had mildly abnormal studies. Nonetheless, another recent large study has shown that 59% of asymptomatic diabetics have abnormal stress MPS studies, including 20% with a “high-risk” scan.49 A further study by this latter group showed that ECG Q waves and/or evidence of peripheral artery disease identified the most suitable diabetic candidates for screening with MPS.50 The differences in these studies is likely explained by differences in underlying risk of the patients studied. Given the diversity of pretest risk in these various diabetic groups, some investigators recommend atherosclerosis testing rather than MPS as a more cost-effective approach to the initial screening tool of diabetics.51,52 A more recent statement from the American Diabetes Association recommended that testing for atherosclerosis or ischemia for patients with type 2 diabetes, perhaps with cardiac CT as the initial test, be reserved for those in whom medical treatment goals cannot be met. Similar recommendations were made for selected asymptomatic diabetics in whom there is strong clinical suspicion of very-high-risk CAD.53
Gender-Based Differences in the Prognostic Value of MPS
The historical limitations of MPS, related to breast tissue artifact and smaller left ventricular chamber size, has been ameliorated in large part with the advent of 99mTc agents. This is related to both enhanced image quality and the ability to gate SPECT images; prone imaging and/or the use of validated attenuation correction algorithms has further aided efforts.36,54
With respect to the prognostic value of MPS, the low risk associated with normal MPS is similar in men and women.8 This is limited, however, in female diabetics where event rates tend to be far greater.21 High-risk findings (e.g., >10% ischemic myocardium) elevated a woman’s risk by nearly 10-fold, with annual rates of major cardiac events of 6.3% for all women and 10.9% for diabetic subsets of women.55
Endothelial dysfunction and microvascular disease have been proposed as mechanisms for false-positive stress testing results, suggesting that some of these studies may represent true perfusion abnormalities without large-vessel CAD. Recent evidence suggests that these MPS perfusion findings may be associated with increased near-term risk of major cardiac events, more so in women than in men,56,57 suggesting that prognostically important coronary disease states not involving obstructive CAD occur more frequently in women than in men, and MPS could provide a tool for detection of this process.
Nuclear Imaging in Elderly Patients
The importance of MPS in an elderly population has grown because of two distinct factors: (1) the aging of the U.S. population and (2) the difficulty in assessing CAD in an elderly population in light of the frequency of asymptomatic and atypical presentations.58,59 This is further confounded by the reduced value of indices such as the DTS60 in an elderly population. Although a relatively smaller proportion of the elderly population is able to achieve adequate exercise on a treadmill, in those who are able to exercise, MPS provides effective risk stratification in elderly men and elderly women.61 This suggests the possibility that exercise MPS may replace ETT as the initial test in an elderly population.
Pharmacologic stress testing is increasingly being applied in the elderly, who frequently are unable to exercise adequately; this population accounts for a high proportion of patients undergoing pharmacologic stress imaging. For elderly patients, as well as for those with functional limitations, similar risk assessment is possible with pharmacologic stress SPECT.62–64 Consistent with data on other functionally impaired patients, the prognostic value of MPS is associated with higher cardiac event rates for normal to severely abnormal test results.
MPS in Patients With Chronic Kidney Disease
There is an increasing recognition of the cardiovascular implications of chronic kidney disease (CKD), and examination of the role of MPS in these patients. CKD is associated with hypertension and dyslipidemia, both promoters of atherosclerosis and further renal damage.65 Because diabetic nephropathy is the leading cause of CKD in the United States, diabetes is often present as well. In addition, CKD is also associated with activation of both inflammatory mediators and the renin-angiotensin system. These factors all contribute to accelerated atherosclerosis and early development of CAD in these patients. Additionally, CKD is associated with worsening risk for the entire spectrum of cardiovascular disease—for example, increased risk of thromboembolism in atrial fibrillation (AF), independent of other risk factors.66 As a result, patients with CKD are exposed to increased morbidity and mortality due to cardiovascular events.65,67 Indeed, the cardiovascular mortality rate in CKD patients is 15 to 30 times the age-adjusted cardiovascular mortality rate in the general population.65,68,69
Determining the role of MPS in this patient population is challenging. On the one hand, successful risk stratification of these patients by MPS results has been reported by a number of investigators.70–74 As we have touched on earlier, post-MPS risk is contextual, resulting in worsening event rates at every level of MPS abnormalities.21,75 In fact, the presence of CKD has been shown to increase risk at any level of MPS results. Hakeem and colleagues followed 1652 patients who underwent stress MPS for more than 2 years, finding that both stress perfusion defects and CKD were independent and incremental predictors of cardiac death after accounting for baseline data, risk factors, left ventricular dysfunction, type of stress used, and symptom status. Hence, MPS results add incrementally and risk-stratify these patients. As important, for any MPS result, normal or abnormal, cardiac mortality is far greater in CKD patients, and the degree of renal dysfunction is predictive of adverse outcome, even after adjusting for MPS data.74 However, in light of the relatively high event rates after a normal MPS, it remains unclear whether and how MPS results can guide the management of CKD patients. Is the risk associated with normal MPS in CKD patients amenable to treatment? Is it lower than the baseline risk of patients with kidney disease in the United States? Does therapeutic action based on abnormal MPS data result in improved patient outcomes?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree