STROKE INTERVENTION
Early reperfusion therapy for acute stroke, similar to that for acute myocardial infarction, offers the best opportunity to reduce the significant morbidity and mortality. Treatment options include intravenous (IV) thrombolysis therapy and/or catheter-based therapy (CBT). Catheter-based therapies include local, intra-arterial administration of thrombolytic agents, mechanical thrombectomy, and percutaneous transluminal angioplasty (PTA) and stent techniques.
Fewer than 2% of acute stroke patients receive any form of reperfusion therapy, and sadly, little is being done to remedy this problem.
1 Acute ischemic stroke is a leading cause of death and disability that will affect three-quarters of a million Americans this year.
2,
3 The therapeutic goal of acute stroke therapy, as with acute myocardial infarction (AMI) therapy, is early reperfusion to minimize end-organ damage. Frustratingly, even among the select centers participating in the Get With The Guidelines-Stroke (GWTG-Stroke) registry, fewer than one-third meet the goal of “door to needle time” of ≤60 minutes.
4While the national quality mandate for a 90 minute doorto-balloon time (D2B) has revolutionized AMI care,
5 there are few hospitals offering “on-demand” catheter-based stroke therapy. Despite the fact that many acute stroke patients present too late (>3 to 4.5 hours) or are poor candidates for IV thrombolysis, many remain eligible for catheter-based reperfusion therapy.
6,
7,
8The nationwide shortage of interventional neuroradiologists is a significant barrier to providing on-demand stroke reperfusion treatment.
9 One solution would be to expand the pool of stroke interventionalists by recruiting carotid artery stent (CAS)-capable physicians, that is, cardiologists, radiologists, vascular surgeons, and neurosurgeons, to join stroke teams.
8,
10,
11 CAS-capable interventionalists, neurointerventionalists, and neurologists should be working together to build stroke teams.
7,
8,
10,
11,
12Ten years ago, the National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Study Group’s randomized trial compared intravenous thrombolysis to placebo therapy in patients presenting within 3 hours of stroke onset and demonstrated a statistically significant but clinically modest benefit for this relatively small subset of acute stroke patients.
13 The NINDS trial found no difference between the two groups’ neurological outcomes at 24 hours, but there was a tenfold higher (6.4% rt-PA versus 0.6% placebo,
P < 0.001) risk of intracranial bleeding with lysis in the rt-PA group. At the 3-month follow-up, a higher percentage of lytic patients (34% lytic versus 21% placebo,
P < 0.05) had achieved full recovery. Unfortunately, there was no difference in mortality between the groups. Ten years after the pivotal trial and despite the fact that IV thrombolysis is the only approved therapy for acute ischemic stroke, no more than 2% of ischemic stroke patients actually receive this treatment.
1,
14Catheter-based therapy for stroke, like intravenous thrombolysis, is not without risks. Intracranial hemorrhage (symptomatic and asymptomatic) rates of 10% to 35% have been reported.
15,
16,
17 Unfortunately, most clinical trials enroll relatively small numbers of patients, providing a small data set upon which to base clinical decision-making. The lack of large clinical trials of acute ischemic stroke treated with CBT makes it difficult to establish guidelines for the “optimal” therapy for stroke.
Illustrative Points
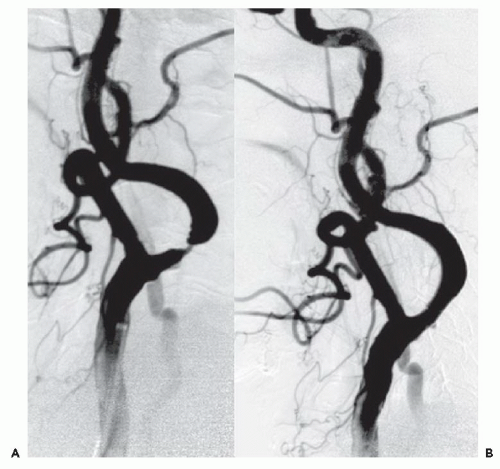
Both patients presented with profound neurologic deficits likely to result in severe disability, and both were dramatically improved after CBT reperfusion therapy. Common features associated with stroke lesions include a large clot burden,
organized thrombus, or embolization of atherothrombotic material that may render these intracerebral occlusions less responsive to thrombolysis than acute thrombi associated with myocardial infarction.
It is important to recognize the similarities and understand the fundamental differences between ischemic stroke and heart attack. Both represent ischemic events, but heart attack is usually the result of plaque rupture and in-situ thrombosis, whereas ischemic strokes more often result from atherothrombotic embolism.
19 The treatment goal for both is safe and rapid reperfusion in order to restore blood flow to the ischemic tissue.
Our second case involved CBT as “rescue” therapy after failure to respond to intravenous thrombolytic therapy. Despite her early presentation, lytic therapy was not successful. Data from NINDS suggest that thrombolytic failure is not infrequent
20 and that large vessel occlusions are less likely to recanalize with lytic therapy alone.
21,
22 Catheter-based therapy with adjunctive mechanical thrombectomy or mechanical clot disruption with angioplasty techniques offers the potential for successfully opening occlusions resistant to thrombolysis.
CBT has become the treatment of choice for large disabling strokes within 6 hours of onset, in patients who are not candidates for IV thrombolytic therapy. With proper patient selection and technique, the outcome of CBT patients who are not candidates for lysis can be as good as that of patients who receive lysis. Recent data has suggested that CBT for large-vessel strokes can be performed by experienced cardiologists working with neurologists and/or neuroradiologists. It is important to remember that the anatomy, pathology, and treatment of acute stroke are different from those of AMI. Intracranial hemorrhage, the most common serious complication of stroke intervention, is usually fatal if it occurs in this setting.
Both cases highlight the magnitude of benefit that can result from CBT in patients with disabling stroke. With the support of a multidisciplinary and cooperative stroke team, qualified physicians can effectively supplement the severe work force shortage of neurointerventionalists.
ELECTIVE INTRACRANIAL ANGIOPLASTY
Approximately 1 in 10 strokes is owing to intracranial atherosclerotic disease,
23 with a higher incidence of the latter seen in Asians
24 and African Americans.
25 The initial treatment for patients with symptomatic >50% intracranial artery stenoses (IAS) is antithrombotic therapy.
26The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial demonstrated that aspirin was the preferred initial therapy over warfarin owing to an increase in adverse events with warfarin. In the WASID trial, 18% of patients had a recurrent stroke within 18 months despite “best” medical therapy, and of these strokes the majority (73%) occurred in the same territory as that of the previous stroke and almost half were disabling.
27 In the Groupe d’Etude des Stenoses Intra-Craniennes Atheromateuses symptomatiques (GESICA) study, symptomatic patients had a 38% risk of a transient ischemic attack (TIA) or stroke at 2 years of follow-up. Surgical extracranial-to-intracranial (EC-IC) bypass failed to reduce the rates of recurrent stroke, and this approach has been abandoned.
28Patients with symptomatic IAS on anti-thrombotic therapy have a 2-year risk that ranges from 1:5 to 1:2 for recurrent stroke.
26,
29,
30 Because of these very high recurrent stroke rates despite “best” medical therapy and the failure of surgical options, the use of catheter-based therapies has been explored.
31 Early case reports and single-center series have suggested the safety and efficacy of intracranial angioplasty with or without stent placement.
12,
32,
33The continuous development of smaller, more flexible, and more deliverable interventional equipment has improved our access to intracranial lesions and lessened the technical challenges associated with intracranial CBT. Three stent systems are currently FDA approved for intracranial use: (i) Neuroform (Boston Scientific, Natick, MA), (ii) Wingspan (Boston Scientific, Natick, MA), and (iii) Enterprise (Codman Neurovascular/Cordis, Raynham, MA). The periprocedural risks of stroke or death with intracranial CBT have averaged about 10% (116/1138) across multiple series
34,
35,
36,
37,
38,
39,
40,
41,
42 with a median of 7.7% reported in a systematic review of 1,177 published procedures.
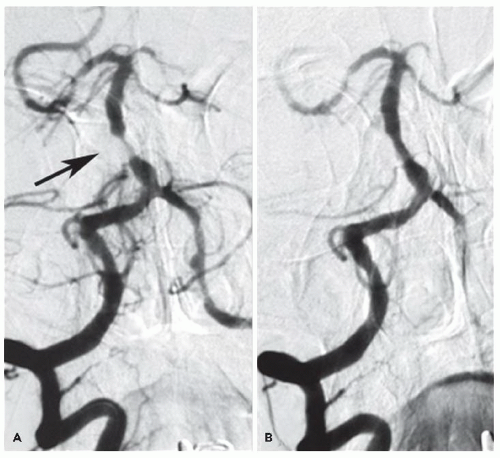
43 A drawback of current stent devices used for IAS is the reported in-stent restenosis (ISR) incidence of 25% to 35%.
44Our experience at the Ochsner Clinic Foundation with CBT in 89 symptomatic patients with 99 (≥70% diameter) IAS treated by interventional cardiologists in cooperation with their neurology and neuroradiology colleagues has demonstrated a procedure success rate of 96/99 (97%) of the lesions and a rate of in-hospital periprocedural stroke and/or death of 3%. Our 1-year (5.7%) and 2-year (13.5%) risk of stroke or vascular death compares very favorably with the results of “best” medical therapy reported in the WASID trial of 15% at 1 year and 20% at 2 years.
26A recent setback for CBT in symptomatic IAS patients was the publication of the Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) clinical trial (gov number NCT00576693). This trial compared intracranial primary stenting performed by neuroradiologists to medial therapy, and was stopped early for safety reasons. After 451 patients underwent randomization, the 30-day rate of stroke or death was 14.7% in the CBT group and 5.8% in the medical-management group
45 (
P = 0.002). Several significant concerns have been raised about the quality of data in this trial, and perhaps some methodological shortcomings that led to such very high periprocedural complication rates include (i) the choice of self-expanding stents (Wingspan, Boston Scientific, Watertown, MA), (ii) prohibition of poststent dilation, and (iii) a concern over the clinical skills of the operators.
46The natural history of patients with severe symptomatic IAS who have failed antithrombotic therapy is not very encouraging, and the relatively low complication rate for CBT in experienced hands makes it a reasonable alternative. However, the recent publication of the large, randomized SAMMPRIS trial should give us reason to redefine our patient selection criteria, rethink stent placement, reevaluate who performs the procedure and include high-volume coronary interventionalists, and refine the procedural techniques to minimize periprocedural complications.
Ultimately, the decision to proceed with CBT for symptomatic IAS patients requires balancing the procedure risks with the natural history of the disease on a case-by-case basis. Patients at the highest risk for recurrent stroke should be selected by lesion severity, lesion location, and documented failure of antithrombotic therapy. The natural history of such patients suggests that the recurrent stroke rates are high (20 to 55%), especially for restenoses
29 (>70%). There is a need for newer, more flexible, self-expanding stents with better coverage and radial force and perhaps a role for drug-coated coronary balloons to improve the outcome of angioplasty in the intracranial circulation.
Illustrative Points
Intracranial intervention, both elective and emergency, is a field in its early development where collaboration between neurology, neuroradiology, and cardiology is important. These cases illustrate how patients with recurrent focal symptoms may benefit from angiography and intervention even if the duplex examination shows no cervical carotid stenosis. Lesions of the aorto-ostial common carotid artery and the intracranial carotid or vertebral arteries can be missed by carotid duplex examination.
CTA and MRA may also under- or overestimate the percent diameter stenosis, so invasive digital subtraction angiography remains the gold standard. Stenting is performed in a provisional manner because (i) many intracranial lesions are not stentable with current devices because of tortuosity in the target vessel, and (ii) side-branch occlusion of the small branches, especially of MCA (lenticulostriate arteries) and the basilar artery (pontine perforators), can lead to a major stroke, which may be more likely with stents than with PTA alone.
AORTIC ARCH AND CERVICAL VESSELS
Extracranial Carotid Artery Intervention
The majority of patients with internal carotid atherosclerotic narrowing are asymptomatic. The annual risk of stroke is between 1% and 4.3% for asymptomatic patients with ≥50% stenosis of the carotid artery.
47,
48 If there is plaque ulceration, the risk of stroke, in asymptomatic patients, increases to 7.5% per year. Symptomatic patients have a worse prognosis as compared to asymptomatic patients.
A transient ischemic attack (TIA), defined in the past as a neurologic event lasting <24 hours, predicted a 15% risk of stroke at 1 month and a 30% risk of TIA, stroke or death within 3 months.
49,
50Large randomized trials begun in the 1990s demonstrated superiority of carotid endarterectomy (CEA) versus antiplatelet therapy (aspirin) for stroke prevention in both symptomatic and asymptomatic patients.
51,
52,
53,
54 Symptomatic patients have much more to gain from revascularization than do asymptomatic patients. This places a premium on reducing perioperative stroke and death to less than 3% in asymptomatic patients who are likely to live for 5 years. For symptomatic patients there was greater benefit with increasing severity of the stenosis above 50% diameter stenosis. However, in asymptomatic patients, the benefit was equal for moderate (60
% to 79%) or severe (80% to 99%) lesions.
55 There is a paradox, however, for very tight lesions, or near-occlusions, defined in the European Carotid Surgery Trial (ECST) as a severe stenosis with evidence of reduced flow in the distal carotid artery and evidence of narrowing of the poststenotic carotid artery. These patients did not benefit from CEA.
53,
56Carotid artery stenting with embolic protection devices began with registry trials in the late 1990s in patients considered to be at increased risk for CEA
57,
58,
59,
60,
61,
62,
63,
64,
65 (
Table 46.1). The Stenting and Angioplasty with Protection in Patients at High
risk for Endarterectomy (SAPPHIRE) trial is the only randomized multicenter trial comparing CEA and CAS in patients at increased risk for surgery.
66 The 1-year primary endpoint for CEA was 20.1% and 12.2% for CAS (ARR 7.9%, 95% CI: 16.4% to 0.7%,
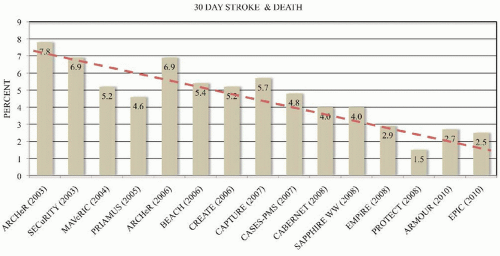
P = 0.004 for noninferiority). Multiple registry trials to obtain FDA approval for CAS systems have shown continued improvement in outcomes with increasing experience (
Figure 46.10). Currently, CAS is the preferred therapy for high surgical risk patients who require revascularization to prevent stroke and who have suitable anatomy for CAS (
Table 46.2).
Four large randomized controlled trials have recently compared CAS to CEA in usual or average surgical risk patients. Three European trials [SPACE,
67 EVA-3S,
68 and ICSS
69] enrolled symptomatic patients with inexperienced CAS operators and who had no requirement for embolic protection devices (EPD). The largest randomized trial to compare CEA to CAS was the Carotid Revascularization Endarterectomy and Stenting Trial (CREST), which was based in the United States and Canada.
70 CREST required stent operators to qualify by experience to gain entry into the trial, required that EPDs be used, and enrolled both symptomatic and asymptomatic patients. CREST enrolled 2,502 average surgical risk symptomatic (53%) and asymptomatic (47%) patients. Patients were followed out up to 4 years without any difference in the rates of the primary endpoint between CAS (7.2%) and CEA (6.8%) with a hazard ratio of 1.11 (95% CI: 0.81 to 1.51;
P = 0.51). There
was an excess of minor strokes with CAS (4.1%) as compared to CEA (2.3%), but there was no difference in major strokes (CAS 0.9% versus CEA 0.7%). The CEA group had twice as many MIs (2.3%) as compared to CAS (1.1%). Cranial nerve palsy occurred in 4.8% of the CEA patients. There was an age effect with younger patients (<69 years) doing better with CAS and older patients doing better with CEA. The recent multisociety guideline document has endorsed CAS as Level I indication for usual-risk symptomatic patients.
71
Illustrative Points
For symptomatic (>50%) and asymptomatic (>70% to 80%) carotid stenosis there are seven CAS systems approved for use by the FDA for high surgical risk patients and one CAS system, for usual surgical risk patients. The most recent multisocietal guideline document makes CAS the preferred treatment for selected high surgical risk patients and a Level I indication for symptomatic >50% diameter stenosis patients.
Self-expanding stents with embolic protection devices are preferred for the carotid bifurcation because of the risk of crushing associated with balloon expandable stents. These stents can be delivered through either a 6F sheath or an 8F guiding catheter. There seems to be no evidence favoring one type of stent over another—closed-cell versus open-cell or stainless steel versus nitinol. There are no data supporting the use of “tapered” stents over nontapered stents in the carotid artery. The choice of delivery mode depends upon the operator’s preference and the presence or absence of tortuosity in the common carotid artery. The nonkinking 6F sheaths have an outer diameter approximately the same as that of an 8F guiding catheter, so the size of the access site hole is similar for both.
The emergence of proximal protection devices has raised the bar for embolic protection. Comparative studies demonstrate less evidence of cerebral embolism with proximal protection systems as compared to distal filters, but proximal protection systems increase the complexity of the case, while filters are very user friendly.
72Unlike in coronary artery stenting, the goal is not a perfect angiographic result after carotid stenting. The main risk of this carotid intervention is distal embolization because of the large plaque burden, so great care is taken not to overdilate the carotid plaque any more than is necessary to achieve a <30% lesion. Many operators will prefer to avoid poststent balloon expansion if possible. Using a technique of underdilation of self-expanding stents, a target lesion revascularization rate of <5% can be expected.
Hypotension and bradycardia with predilation of the carotid stenosis is a marker for hemodynamic instability with stent placement owing to stimulation of the carotid body. Hypotension occurring hours following carotid stenting may signify access site bleeding. This generally responds to volume expansion, although atropine and alpha agonists such as neosynephrine and midodrine are employed when volume expansion alone is not sufficient.
Vertebral Artery Intervention
Approximately 20% to 25% of ischemic strokes involve the posterior circulation and the vertebrobasilar system.
73,
74,
75 The prognosis for patients with posterior circulation atherosclerotic disease is poor, with occlusion or thrombosis associated with 80% to 100% mortality.
76 Medically refractory, symptomatic vertebral artery disease carries a 5% to 11% incidence of stroke or death at 1 year.
77,
78,
79,
80 Transient ischemic attacks owing to extracranial vertebral disease are associated with a stroke rate of 30% at 5 years.
80,
81,
82Revascularization of the vertebral arteries is indicated only for symptomatic patients. Typical symptoms include vertebrobasilar insufficiency (VBI), namely, dizziness, ataxia, visual disturbances, confusion, or coma. When assessing a patient with significant stenosis of a vertebral artery, the interventionalist should always determine the patency of the contralateral vertebral artery, the dominance of the diseased vertebral artery, and the amount of blood supplied to the vertebrobasilar system by the carotid arteries.
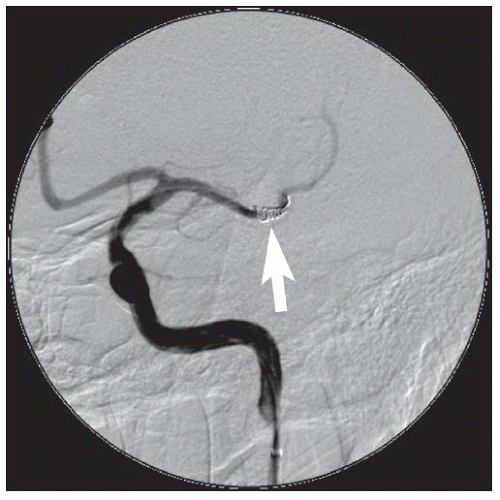
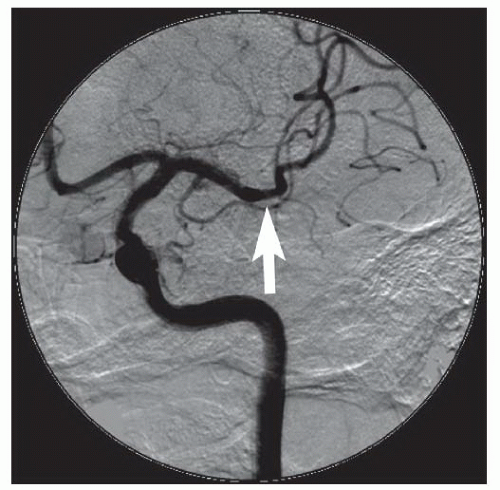
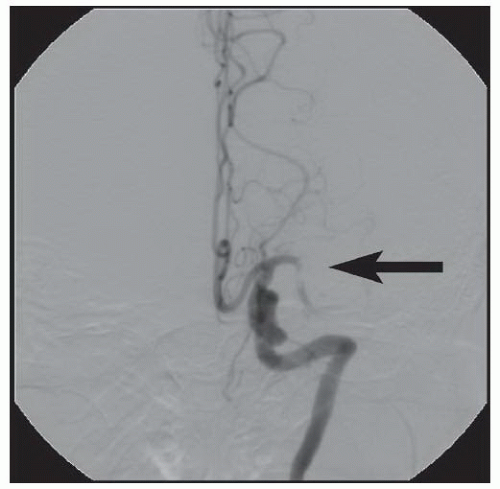
Depending upon the location of the stenosis, treatment options for vertebral artery stenosis may include balloon angioplasty with or without stent placement
83 and surgery involving transplantation of the vertebral arteries onto the carotid artery or bypass grafting from the subclavian to the vertebral artery.
84Lesions located at the ostium (V
0) or proximal (V
1), mid (V
2-3), or distal (V
4) segments of the vertebral artery can be approached percutaneously.
83,
85 We prefer primary balloonexpandable stent placement for V
0 and V
1 lesions to scaffold the lesions and minimize elastic recoil. Lesions located more distally may be treated with balloon angioplasty with provisional balloon-expandable or self-expanding stents, depending upon the angiographic results and the tortuosity of the vessel. Lesions at the vertebrobasilar junction (V
4) and in the basilar artery have a higher complication rate and are the most prone to dissection, occlusion, and perforation, and stroke.
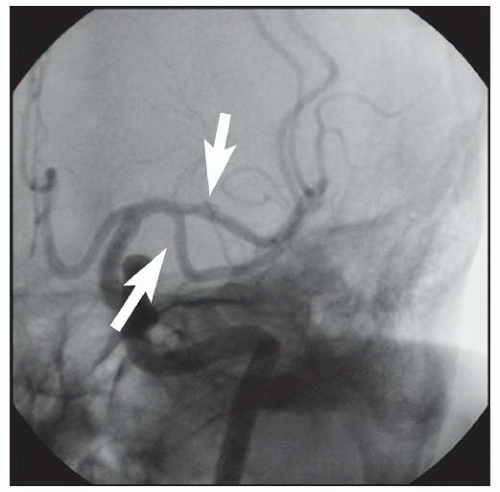
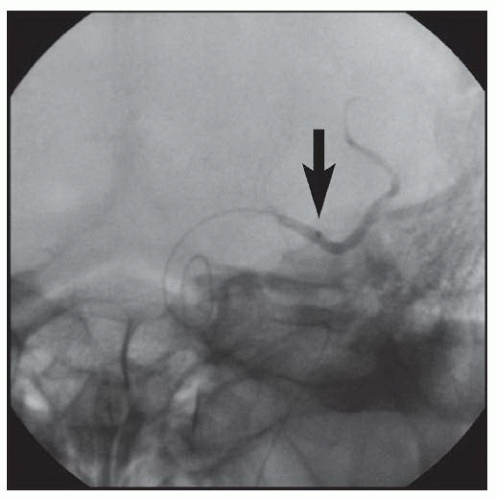
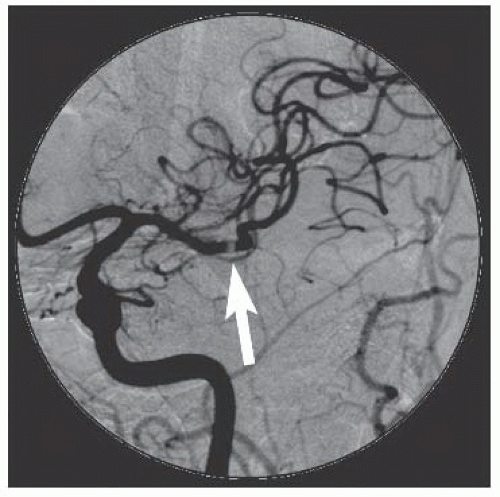
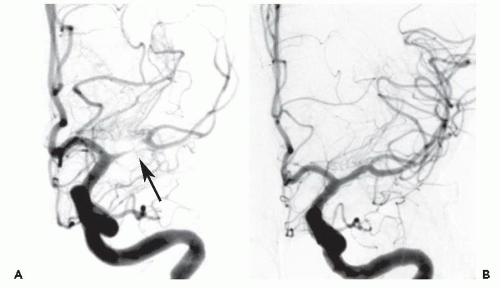
86We have recently reported the largest single-center series of vertebral artery intervention in 105 consecutive
symptomatic patients
83 (112 arteries, 71% male). Technical and clinical success was achieved in 105 (100%) and 95 (90.5%) patients, respectively. One-year follow-up was obtained in 87 (82.9%) patients, of which 69 patients (79.3%) remained symptom-free. At 1 year, six patients (5.7%) had died and five patients (5%) had a posterior circulation stroke. Target vessel revascularization (TVR) occurred in 7.4% at 1 year. At a median follow-up of 29.1 months (IQR 12.8 to 50.9 months), 71.4% were alive, 13.1% underwent TVR, and 70.5% remained symptom-free. Our data demonstrate that vertebral stent placement in symptomatic patients in experienced hands can be accomplished with a very high success rate (100%), with few periprocedural complications, and is associated with durable symptom resolution. We concluded that stenting of atherosclerotic vertebral artery disease is a safe and effective treatment and should be considered first-line therapy for this disease.




 High cervical or intrathoracic lesion
High cervical or intrathoracic lesion Age ≥80 y
Age ≥80 y Prior radical neck surgery or radiation
Prior radical neck surgery or radiation Class III/IV congestive heart failure
Class III/IV congestive heart failure Contralateral carotid artery occlusion
Contralateral carotid artery occlusion Class III/IV angina pectoris
Class III/IV angina pectoris Prior ipsilateral CEA
Prior ipsilateral CEA Left main coronary disease
Left main coronary disease Contralateral laryngeal nerve palsy
Contralateral laryngeal nerve palsy Multivessel coronary artery disease
Multivessel coronary artery disease Tracheostoma
Tracheostoma Planned surgery (<30 days)
Planned surgery (<30 days) LV ejection fraction ≤ 30%
LV ejection fraction ≤ 30% Recent (≤30 d) myocardial infarction
Recent (≤30 d) myocardial infarction Severe lung disease
Severe lung disease Severe renal disease
Severe renal disease Age ≥80 y
Age ≥80 y Complex aortic arch
Complex aortic arch Operator inexperience
Operator inexperience Symptomatic
Symptomatic Tortuosity
Tortuosity No emboli protection device
No emboli protection device Decreased cerebral reserve
Decreased cerebral reserve Calcification
Calcification Time delay from symptom onset
Time delay from symptom onset Hypercoagulable state
Hypercoagulable state Intraluminal thrombus
Intraluminal thrombus Open cell stents
Open cell stents Severe renal disease
Severe renal disease Echo lucent plaque
Echo lucent plaque Vascular access difficulty
Vascular access difficulty Increased bleeding risk
Increased bleeding risk