Profiles in Pericardial Disease
John F. Robb
Roger J. Laham
Mauro Moscucci
Drs. Beverly Lorrell and William Grossman contributed material for this chapter in previous editions.
Pericardial disease can manifest as fluid accumulation owing to injury or inflammation (pericardial effusion, possibly leading to pericardial tamponade) or as progressive thickening of the parietal and/or visceral pericardium (possibly leading to pericardial constriction). Both tamponade and constriction impede diastolic filling, elevate right and left heart diastolic pressures, and reduce cardiac output, but these two processes differ significantly in the pattern of diastolic filling impairment during each cardiac cycle and in the hemodynamic response to respiration. There are thus distinctive echocardiographic and hemodynamic profiles for tamponade versus constriction, and for constriction as opposed to restrictive cardiomyopathy, in which impaired left ventricular diastolic filling is owing to reduced myocardial compliance without pericardial involvement. For a detailed description of the hemodynamic characteristics of tamponade, and constrictive and restrictive physiology, the reader is referred to Chapter 23. In this chapter, we review profiles associated with pericarditis, pericardial tamponade, pericardial constriction, and restrictive cardiomyopathy.
PERICARDITIS, PERICARDIAL EFFUSION, AND TAMPONADE
Discussion of the myriad causes of pericarditis and pericardial effusion is beyond the scope of this chapter, but suffice it to say that virtually any pathologic process can affect the pericardium1 and cause a detectable pericardial effusion whenever the rate of accumulation of pericardial contents (transudate, exudate, pus, blood, or gas) exceeds the reabsorption ability of the pericardium. The most common causes of pericardial effusion include idiopathic pericarditis (presumably viral), trauma (including iatrogenic catheter injury to the cardiac chambers or vessels), malignancy, postmyocardial infarction, uremia, connective tissue disease, autoimmune disorders, myxedema, and radiation.2,3 Infectious or purulent pericarditis is caused most commonly by Staphylococcus aureus, followed by other Gram-positive organisms, fungi or mycobacteria.4,5 Myocarditis and pericarditis frequently coexist, and elevations of cardiac troponin levels can be seen in both.6 Acute pericardial injury of any type can also trigger an autoimmune process that can lead to continued or recurrent effusion.
Patients with acute pericarditis often experience sharp, aching, or pressurelike precordial pain that is worsened by cough, inspiration, and recumbency. Pain may radiate to the shoulders7 and may be confused with angina or acute myocardial infarction, particularly since the ECG may show diffuse concave ST-segment elevation. One distinguishing feature, however, is the concomitant depression of the PR segment and the absence of reciprocal ST depression. A pericardial friction rub and fever may be present, but rigors or spiking fevers should raise the concern of purulent pericarditis. The mainstay of diagnosis is echocardiography, which clearly shows an effusion. Fluid first accumulates posteriorly and then extends anteriorly. Small effusions have <10-mm clear space between the heart and parietal pericardium, moderate effusions have a 10- to 20-mm gap, and large effusions have a >20-mm gap. The size of the effusion correlates roughly with prognosis.8 Effusions may be loculated (partitioned) by nonuniform fibrous adhesions that form between the parietal and visceral pericardium, a pattern typically observed after cardiac surgery. Echocardiography also reveals the extent to which the pericardial pressure is compromising cardiac function, showing early diastolic collapse of the right ventricle as pericardial pressure transiently exceeds intracavitary pressure. In idiopathic pericarditis, the effusion usually resolves spontaneously or after symptomatic treatment with nonsteroidal anti-inflammatory agents and sometimes with colchicine9 or corticosteroids.
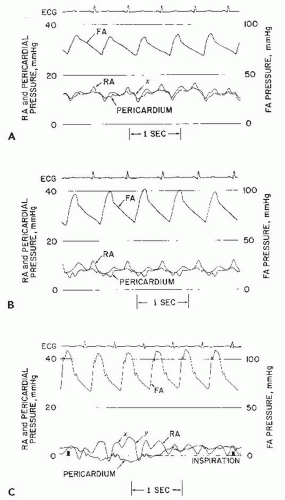
Chronic idiopathic effusions may also persist without symptoms or signs of tamponade despite effusion, and volumes >500 mL may be followed conservatively with serial echocardiograms if asymptomatic.10 On the other hand, significant pericardial effusion with even early signs of hemodynamic compromise is usually an indication for prompt drainage (pericardiocentesis, or surgical subxiphoid window placement). The pericardial pressure is normally subathmospheric (−5 to +5 mmHg) and it tracks the intrathoracic pressure during the inspiratory/expiratory cycle. As discussed in details in Chapter 23, during inspiration the pericardial pressure falls more than the systemic venous pressure. The net result is an increase in right atrial filling gradient leading to augmentation of atrial filling and right ventricular stroke volume. If an excess of pericardial fluid accumulates in the pericardial space beyond its limited capacity to stretch, the pericardial pressure rises and begins to progressively compress the underlying cardiac chambers. The diastolic y descent becomes absent, owing to impairment of rapid atrial emptying secondary to compression by the pericardial effusion. By the time the classic bedside findings of jugular venous distension and pulsus paradoxus (fall in systolic arterial pressure on normal inspiration) develop, only a small further accumulation of fluid separates the patient from frank hemodynamic collapse. As shown in Figure 44.1, the abnormal physiology of cardiac tamponade is relieved when (a) the pericardial pressure falls to a level ≤0 mmHg and separates from the right atrial pressure and (b) the right atrial pressure falls to the normal range and exhibits return of a normal diastolic y descent, indicating restoration of normal rapid atrial emptying and early ventricular diastolic filling.
DIAGNOSTIC AND THERAPEUTIC PERICARDIOCENTESIS
The main pericardial procedure performed in the catheterization laboratory is needle puncture and catheter drainage of pericardial fluid—pericardiocentesis (Chapter 38). Diagnostic pericardiocentesis may be performed to evaluate the etiology of pericarditis, particularly for suspected purulent or tuberculous pericarditis, persistence or recurrence of a large effusion, or a high suspicion of malignant effusion without a tissue diagnosis from the primary site. The diagnostic yield for pericardial fluid analysis is quite low, with as few as 6% of diagnostic procedures and 29% of therapeutic procedures yielding useful information.11, 12, 13 Diagnostic yield tends to be higher in large pericardial effusions.14
Pericardial fluid always should be sent for cell count; Gram, acid-fast bacteria (AFB), and special stains; cultures (aerobic, anaerobic, AFB, fungal); and cytology. Although differentiation between an exudate and a transudate can be accomplished with determination of fluid protein and LDH levels, such differentiation is of dubious value because the timing of sampling and effects of treatment may alter values and because sensitivity and specificity are poor with significant overlap between the exudative and transudative groups.13 Since most effusions are serosanguineous or hemorrhagic, fluid appearance is not useful in differentiating various etiologic groups with the exception of purulent fluid, which is specific but not sensitive for infection. As long as adequate samples are obtained, fluid cytology has 92% to 95% sensitivity and 100% specificity for malignant pericardial effusion, although nonmalignant effusions are also common in patients with underlying malignancy. There are no specific pericardial fluid findings for postpericardiotomy syndrome, radiation or uremic pericarditis, hypothyroidism, or trauma. Special circumstances may require additional analysis for the following: viral cultures for viral infection; fluid cholesterol level in myxedema; fat studies for chylopericardium; latex fixation for rheumatoid antigen; gamma globulin complexes and fluid complement levels for rheumatoid arthritis; fluid antinuclear antibody levels for systemic lupus erythematosus; and tuberculosis stains and/or cultures, fluid adenosine deaminase determination,15 or polymerase chain reaction16 for tuberculosis (see also Chapter 38). Purulent pericarditis may be associated with low pH, high protein levels, glucose levels <35 mg/dL, and elevated leukocyte counts in the range of 6,000 to 240,000/mm3.4,17
PERICARDIAL BIOPSY
The diagnostic yield of pericardiocentesis may be increased by retrieval of pericardial tissue by a surgical pericardial biopsy performed via thoracotomy, subxiphoid incision, or thoracoscopy. Percutaneous pericardial biopsy (assisted by pericardioscopy) has led to a specific diagnosis in 49% to 53% of patients in several limited series.18,19 The pericardioscopy technique is not widely available and requires surgical or 16F percutaneous pericardial access. In hemorrhagic effusion, obtaining adequate parietal pericardial visualization may require 2 to 3 days of active drainage, replacement of pericardial effusion with saline, and instillation of 100 to 300 mL of air.19 Several small uncontrolled series suggest that this approach may increase the likelihood of obtaining a definitive diagnosis in patients with large recurrent pericardial effusions and in patients in whom there is a strong clinical suspicion of malignant pericarditis or tuberculous pericarditis. In a prospective series of 141 patients with unexplained pericardial effusions who underwent 142 surgical pericardioscopy including cytological fluid analysis, visualization of the pericardium, and guided biopsy, a specific etiologic cause (neoplastic, infected purulent, or sterile radiation-induced effusion) was identified in 49%, whereas 51% were considered idiopathic. Of note, an unrecognized cause not detected by pericardioscopy-biopsy was subsequently discovered in 4%.19 No deaths were attributable to pericardioscopy but in-hospital mortality was 5.6% related to underlying disease.
In another series, 35 patients with pericardial effusion owing to inflammatory disease underwent pericardioscopy, and pericardial biopsies were performed. Diagnoses of viral pericarditis, lymphocytic perimyocarditis, bacterial pericarditis, and antibody-mediated autoreactive pericarditis were obtained; however, it was unclear if this resulted in a change of management strategy.20 The same authors reported a series of 14 patients with idiopathic pericarditis
and 15 patients with malignant pericarditis who underwent percutaneous pericardioscopy with both pericardial and epicardial biopsy from a registry of 136 patients undergoing pericardiocentesis. In this study, subxiphoid pericardiocentesis and sampling of fluid for cytology, immunologic studies, and culture were performed first, followed by evacuation of pericardial fluid, placement of warmed clear sterile saline in the pericardial sac, and introduction of both rigid and flexible pericardioscopes. Both epicardial biopsies and pericardial biopsies were obtained with a resterilizable bioptome, after site selection by both pericardioscopy and biplane fluoroscopy, before the saline was evacuated. In this series of patients with proven malignant pericarditis, fluid cytology was diagnostic in 71% and epicardial biopsy in 80%.21
and 15 patients with malignant pericarditis who underwent percutaneous pericardioscopy with both pericardial and epicardial biopsy from a registry of 136 patients undergoing pericardiocentesis. In this study, subxiphoid pericardiocentesis and sampling of fluid for cytology, immunologic studies, and culture were performed first, followed by evacuation of pericardial fluid, placement of warmed clear sterile saline in the pericardial sac, and introduction of both rigid and flexible pericardioscopes. Both epicardial biopsies and pericardial biopsies were obtained with a resterilizable bioptome, after site selection by both pericardioscopy and biplane fluoroscopy, before the saline was evacuated. In this series of patients with proven malignant pericarditis, fluid cytology was diagnostic in 71% and epicardial biopsy in 80%.21
In a more recent study by Seferovic, 49 patients with a large pericardial effusion underwent parietal pericardial biopsy using fluoroscopy or pericardioscopy guidance (for more extensive sampling). Diagnostic efficiency was improved by extensive sampling (4-20 biopsies) as compared with a single biopsy19 (40% versus 8.3%, P < 0.05). In our experience and in several series of pericardiocentesis in patients with malignant effusion, cytologic examination is positive in about 80% to 85% of cases and false-negative cytologic analysis is rare in carcinomatous pericarditis (as opposed to malignant involvement by lymphoma or mesothelioma). In the era of molecular diagnostic tools, a clear role for diagnostic pericardioscopy and directed pericardial and/or epicardial biopsy is not yet defined relative to cytologic examination of recovered fluid.
Therapeutic pericardiocentesis is indicated for any sign or symptom of tamponade, limiting dyspnea,14 symptoms of compression of surrounding structures such as lung or esophagus, and acute hemopericardium with circulatory compromise following a catheter-based or surgical intervention. The diagnosis of early tamponade is usually made echocardiographically. Hemodynamically, the diagnosis of tamponade is made if there is identical elevation of left- and right-side diastolic pressures with loss of the y descent in a patient with pericardial effusion. However, it may be noted that pericardiocentesis is contraindicated for hemopericardium or tamponade in the presence of a diagnosed ascending aortic dissection, as it can accelerate bleeding and shock,22 making immediate surgery preferable in such situations. Another particular example in which emergency pericardiocentesis is required is when catheter injury to a cardiac chamber or vessel produces acute hemopericardium. This may happen as the result of temporary pacemaker placement in the right ventricle, passage of a stiff right heart catheter, endomyocardial biopsy, trans-septal puncture, retrograde crossing of a stenotic aortic valve with a straight guidewire, coronary atherectomy or high-pressure stent dilation, or passage of a stiff hydrophilic guidewire into a small coronary branch in a patient receiving a glycoprotein IIb/IIIa platelet antagonist.
The patient may complain of chest pain, followed by progressive hypotension and tachycardia. Bradycardia may also appear owing to stimulation of the vagal nerve by blood in the pericardium, but the hypotension persists even after the bradycardia is resolved by atropine administration. Careful fluoroscopy of the right and left heart borders may show that the normal pulsations of the heart have been replaced by an immobile tense pericardial shadow, and right heart catheterization may show the classic hemodynamic findings described below as well as inability to advance the right heart catheter fully to the right atrial border. Urgent pericardiocentesis may be lifesaving in this situation. When the cause of bleeding into the pericardium is injury to a coronary artery, either placement of a fabric-covered stent or coil embolization of a small bleeding distal branch may be required. Ongoing bleeding after initial drainage and reversal of anticoagulation, however, usually warrants emergency surgery.
While percutaneous pericardiocentesis is the procedure commonly used for acute pericardial tamponade, surgical or balloon pericardiotomy is performed for recurrent effusions. The techniques of pericardiocentesis, balloon pericardiotomy, and the pericardial approach to epicardial ablation for ventricular arrhythmias and for left atrial appendage exclusion are described in Chapter 38.
CASE STUDIES
CASE 44.1 Pericardial Tamponade.
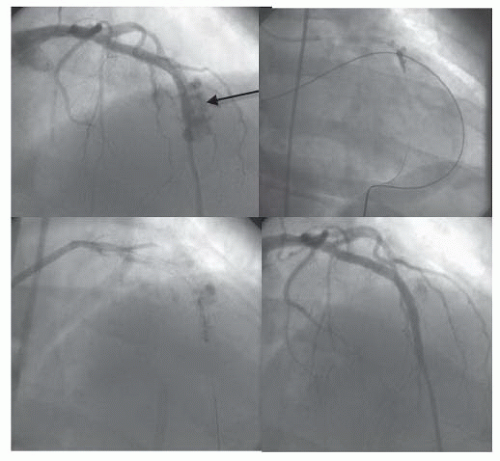
A 55-year-old man presented with unstable angina. Severe stenoses in the proximal and mid LAD were stented, but a shelflike stepup was seen within the stented segment. That area was postdilated at 16 atm, with vessel perforation noted immediately on deflation (Figure 44.2). Hypotension ensued, and the 3.0-mm balloon was reinflated to block the vessel as emergency subxiphoid pericardiocentesis was performed. The contralateral femoral artery was punctured to allow introduction of an 8F XB3.5 guiding catheter, through which a second BMW wire was advanced to the distal vessel. The 3.0-mm balloon was deflated and removed through the initial 6F guiding catheter as a 3.0 × 16 mm Jomed covered stent was advanced to span the area of perforation. Delivery of the stent and postdilation at 18 atm sealed the perforation. Cardiac surgeons were present in the catheterization laboratory, who agreed with continued nonoperative management, given that bleeding had been stopped and the vessel was patent. No further pericardial problems were noted, and the drain came out the next day. The patient did have moderate myocardial infarction (MI) owing to occlusion of diagonals, but the stent remained open at restudy on day 3, and the patient went home on day 4.
CASE 44.2 Balloon Pericardiotomy for Recurrent Effusion.
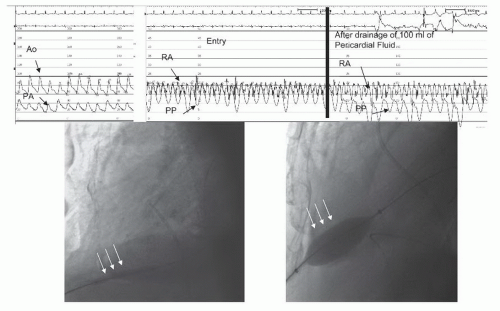
A 54-year-old man with lung adenocarcinoma that had been treated with chemotherapy, radiation therapy, and right lobectomy with chronic left pleural effusion (chest tube in place), developed a large pericardial effusion with echocardiographic evidence of pericardial tamponade (right atrial and right ventricular collapse). He underwent pericardiocentesis showing a prepocedure pericardial pressure of 23 mmHg, with relief of tamponade physiology after drainage of more than 500 mL of serosanguineous fluid. Two weeks later, however, symptoms recurred and echocardiography showed reaccumulation of pericardial effusion and signs of tamponade. Right heart catheterization showed a mean right atrial pressure of 16 mmHg, mean PCW pressure of 22 mmHg, and mean pericardial pressure of 15 mmHg with some separation of pericardial and right atrial pressures. Cardiac index was mildly depressed at 2.4 L/minute/m2, with a 15 mmHg paradox.
After needle pericardiocentesis and removal of 100 mL of bloody fluid, the pericardial pressure decreased to 0 mmHg with little change in the right atrial (RA) pressure. Balloon pericardiotomy (Figure 44.3) was performed by advancing a guidewire through the drainage catheter, predilating with a 4.0 × 40 mm balloon, and final dilation with inflation of a 15 × 40 mm balloon straddling the pericardial edge. The pericardial waist resolved fully, and the balloon was exchanged for a drainage catheter, which was left in place overnight. The cardiac index improved to 3.4 L/minute/m2, and repeat echocardiography at 1 month showed no reaccumulation of pericardial fluid.
CASE 44.3 Pericardial Effusion in a Patient with Systemic Sclerosis.
A 38-year-old woman was admitted to the hospital with progressive dyspnea. Her past medical history was significant for systemic sclerosis and pulmonary hypertension. Initial echocardiographic evaluation revealed a large pericardial effusion. There were no echocardiographic indications of cardiac tamponade. The left ventricle was hyperdynamic, the right ventricle was moderately dilated, and the right ventricular systolic performance was severely reduced. The right ventricular systolic pressure was estimated at 60 mmHg. Owing to the presentation with progressive dyspnea and concerns that the large pericardial effusion was contributing to the patient’s symptoms, she was referred for right heart catheterization and possible pericardiocentesis. The mean right atrial pressure was 11 mmHg, the right ventricular pressure was 80/15 mmHg, the pulmonary artery pressure was 78/35 mmHg with a mean of 45 mmHg, and the mean pulmonary capillary wedge pressure was 30 mmHg. Pericardial pressure was 10 mmHg. Cardiac output was 4.14 L/minute and the cardiac index was 2.55 L/minute/m2. A total of 50 cc of pericardial fluid was removed, and the patient was transferred to the CCU with a pericardial drain in place. Over the next 2 days, a total of 600 cc was slowly drained at a rate of 100 cc every 8 hours. Repeat hemodynamic measurements in the CCU revealed a decrease of the pulmonary capillary wedge pressure to 8 mmHg, while the pulmonary artery systolic pressure was 70 mmHg. Her dyspnea was resolved. A pericardial rub developed on the third postprocedure day. The patient was started on prednisone as well as on sildenafil. She continued to improve, and on the day of discharge she was able to tolerate a 6 minute walk test without development of dyspnea or oxygen desaturation.
Clinical Considerations. Pericardial effusion is a relatively common finding in late stages of pulmonary hypertension. The typical findings of pericardial tamponade including right atrial diastolic collapse, right ventricular diastolic collapse, and changes in Doppler transmitral flow velocity tend to be absent in this setting. Importantly, poor outcomes including
a high mortality rate have been observed in patients with pulmonary hypertension or systemic sclerosis undergoing drainage of large pericardial effusions.23,24 It should be noted that acute left and right ventricular failure are known complications of pericardiocentesis. The pathophysiology of acute left and right ventricular failure is unclear (see Chapter 38 for a detailed description of available hypotheses), although there exists a general agreement that rapid drainage of large amounts of fluid can contribute to its development. Whether the high mortality rate observed after drainage of pericardial effusions in patients with pulmonary hypertension is secondary to acute left and right ventricle dysfunction remains to be determined, although it is a plausible hypothesis. Therefore, in this case meticulous attention was paid to avoid drainage of a large amount of fluid in a short period of time.
a high mortality rate have been observed in patients with pulmonary hypertension or systemic sclerosis undergoing drainage of large pericardial effusions.23,24 It should be noted that acute left and right ventricular failure are known complications of pericardiocentesis. The pathophysiology of acute left and right ventricular failure is unclear (see Chapter 38 for a detailed description of available hypotheses), although there exists a general agreement that rapid drainage of large amounts of fluid can contribute to its development. Whether the high mortality rate observed after drainage of pericardial effusions in patients with pulmonary hypertension is secondary to acute left and right ventricle dysfunction remains to be determined, although it is a plausible hypothesis. Therefore, in this case meticulous attention was paid to avoid drainage of a large amount of fluid in a short period of time.
CONSTRICTIVE PERICARDITIS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree