Profiles in Coronary Artery Disease
Robert N. Piana
Aaron Kugelmass
Jeffrey J. Popma and Judith L. Meadows authored this chapter in the prior edition.
Atherosclerotic coronary artery disease (CAD) continues to be the most frequent cause of death in the United States and other developed nations.1, 2, 3 Besides mortality, coronary artery disease accounts for substantial morbidity and disability. Diagnostic and therapeutic procedures for coronary disease have evolved rapidly over the last 40 years, and in parallel with advancements in medical therapy, have resulted in a significant decrease in both morbidity and mortality.4 The medical and procedural progress in the treatment of CAD represents one of the major accomplishments of modern medicine.
Cardiologists play a crucial role in identifying clinical CAD and developing a cogent treatment plan for an individual patient. The cardiovascular physician is charged with applying evidence and guideline-based diagnostic and treatment regimens that are individualized around anatomic and clinical characteristics. Though technical in basis, these approaches must also consider patient and family preference, and thus incorporate cultural, emotional, and value-based considerations on a background of clinical science.
This chapter is designed to provide examples of patient-centered therapy of CAD based on individual clinical and angiographic profiles. These case-based examples have been selected to demonstrate the application of clinical evidence and guideline recommendations of percutaneous coronary intervention (PCI) in the contemporary management of CAD.5
STABLE CORONARY ARTERY DISEASE
In patients with symptoms of stable angina, it is critical to establish a diagnosis of coronary artery insufficiency. While this may be based solely on functional noninvasive testing, coronary angiography using cardiac catheterization6 and, in selected cases, coronary computed tomographic angiography (CTA)7 are indicated in patients with high-risk functional testing, or in whom diagnostic certainty is critical.6 The objective of therapy for stable CAD is to reduce not only mortality, but also prevent further progression, anginal pain, and disability.
For the majority of patients with clinically stable, symptomatic CAD, guideline-directed medical therapy (GDMT),8 including aspirin, beta blockade, hypertension control, and HMG-coA reductase inhibitors (statin) if tolerated, and lifestyle modification constitute the primary proven treatment modality at this time.9,10 In advanced CAD, significant left main CAD, and three-vessel CAD with diminished left ventricular systolic function, surgical revascularization with coronary artery bypass graft surgery (CABG) has demonstrated survival benefit over historic (limited) medical therapy.
The Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial randomized patients with stable coronary artery disease (single-vessel and low-risk multivessel) to GDMT versus GDMT and PCI.11 This trial demonstrated no significant reduction in cardiac mortality, myocardial infarction, need for revascularization, or long-term angina symptoms in those patients treated with PCI. These trial findings have remained controversial, but pending more contemporary trials that utilize advanced imaging techniques and drug-eluting stents (DESs), an initial therapeutic approach of guideline-directed medical therapy (GDMT) has been deemed appropriate.8,10 More recently, the Fractional Flow Reserve-Guided PCI versus Medical Therapy in Stable Coronary Disease (FAME 2) trial suggested that in patients with stable CAD, FFR-guided PCI of lesions (FFR < 0.80) in addition to optimal medical therapy can reduce the incidence of urgent revascularization.12 Whether this approach will be adopted in clinical guidelines or practice remains to be determined.
For those patients who continue to experience lifestyle limiting angina despite guideline-directed medical therapy, coronary revascularization is an option. For patients with single-vessel CAD, PCI is an option and class I indication8 when GDMT fails in relieving symptoms. For patients with
multivessel CAD, PCI and bypass surgery have been shown to have similar 5-year rates of myocardial infarction and death.13 However, the need for repeat revascularization is higher in patients undergoing PCI. Stratification of multivessel CAD patients for PCI on the basis of angiographic complexity can, however, select patients in whom this risk is minimal.14 In addition, a note of caution should be applied regarding the choice of revascularization for the subgroup of patients with diabetes mellitus. The Bypass Angioplasty Revascularization Investigation (BARI) trial suggested a survival benefit of CABG when compared to PCI in patients with multivessel disease and diabetes mellitus, thus raising an initial concern in this patient population.15 This concern has been confirmed by several subsequent clinical trials and registry analyses and by a meta-analysis summarizing 10 randomized clinical trials.16 More recently, in the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial, patients with diabetes mellitus and multivessel coronary artery disease were randomized to revascularization with CABG or with contemporary PCI utilizing drug-eluting stents.17 The primary outcome was a combined endpoint including death from any cause, nonfatal myocardial infarction, and nonfatal stroke at 5 years. At 5-year follow-up, the primary endpoint occurred more frequently in patients undergoing PCI when compared to patients undergoing CABG. The difference between PCI and CABG was driven by a higher rate of death from any cause and nonfatal myocardial infarction in the PCI group when compared to the CABG group.17 Thus, when considering revascularization for patients with multivessel CAD, the revascularization modality should be established on the basis of patient preference, clinical and angiographic characteristics that are determinant of acute and long-term success, and the presence of diabetes mellitus. As such, for patients with multivessel CAD requiring revascularization, collaborative, evidence-driven decision-making by cardiologists and cardiac surgeons, based on clinical and angiographic determinants of acute and long-term benefit, is essential.8
multivessel CAD, PCI and bypass surgery have been shown to have similar 5-year rates of myocardial infarction and death.13 However, the need for repeat revascularization is higher in patients undergoing PCI. Stratification of multivessel CAD patients for PCI on the basis of angiographic complexity can, however, select patients in whom this risk is minimal.14 In addition, a note of caution should be applied regarding the choice of revascularization for the subgroup of patients with diabetes mellitus. The Bypass Angioplasty Revascularization Investigation (BARI) trial suggested a survival benefit of CABG when compared to PCI in patients with multivessel disease and diabetes mellitus, thus raising an initial concern in this patient population.15 This concern has been confirmed by several subsequent clinical trials and registry analyses and by a meta-analysis summarizing 10 randomized clinical trials.16 More recently, in the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial, patients with diabetes mellitus and multivessel coronary artery disease were randomized to revascularization with CABG or with contemporary PCI utilizing drug-eluting stents.17 The primary outcome was a combined endpoint including death from any cause, nonfatal myocardial infarction, and nonfatal stroke at 5 years. At 5-year follow-up, the primary endpoint occurred more frequently in patients undergoing PCI when compared to patients undergoing CABG. The difference between PCI and CABG was driven by a higher rate of death from any cause and nonfatal myocardial infarction in the PCI group when compared to the CABG group.17 Thus, when considering revascularization for patients with multivessel CAD, the revascularization modality should be established on the basis of patient preference, clinical and angiographic characteristics that are determinant of acute and long-term success, and the presence of diabetes mellitus. As such, for patients with multivessel CAD requiring revascularization, collaborative, evidence-driven decision-making by cardiologists and cardiac surgeons, based on clinical and angiographic determinants of acute and long-term benefit, is essential.8
Invasive, as well as CT based, coronary angiography is effective in identifying fixed stenosis of coronary arteries. While important in establishing a diagnosis of CAD with attendant need for secondary prevention, it is the functional significance of individual coronary stenosis that is critical in developing a patient-centered therapeutic strategy. Physiologic stress testing, both exercise and pharmacologic, provides the physiologic basis for CAD treatment, especially revascularization. Similarly, fractional flow reserve (FFR) can provide critical functional information in the cardiac catheterization lab. This invasive technique (see Chapter 24) provides the opportunity to further assess the functional significance of specific coronary stenoses in order to direct therapy at the time of angiography. FFR has been shown to be similar to perfusion stress testing in predicting clinical events associated with a given stenosis. Conversely, FFR has been shown to be effective in identifying coronary stenoses that do not require revascularization in order to prevent CAD-related events.12,18,19 Like all procedures, FFR should be used judiciously. For those patients with a severe stenosis that corresponds to a territory of ischemia identified with functional testing, there is no need to perform FFR. However, in the case of intermediate stenoses, or stenoses that do not appear to be related to ischemia by functional testing, FFR should be performed in order to assess functional significance.
CASE STUDIES
CASE 41.1
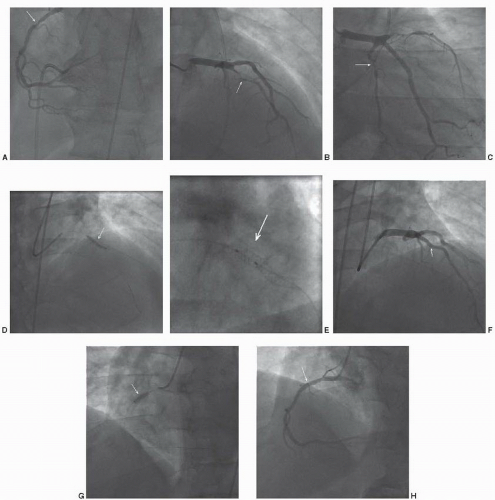
A 45-year-old man with a history of hypertension, hyperlipidemia, and cigarette smoking presented to his physician with exertional dyspnea and vague chest discomfort. He was being treated with a statin, beta blockers, and an ACE inhibitor. Exercise stress testing with sestamibi scintigraphy, to 9 METS, demonstrated severe reversible perfusion defects of the inferior, inferoapical, anterior, and anteroapical segments. Rest ejection fraction was 52%, but declined to 33% during stress. There was transient left ventricular dilation. Nitrates and aspirin were added to his medical regimen. The patient was referred for coronary angiography (Figure 41.1). This demonstrated severe three-vessel CAD, with discrete lesions in the proximal right coronary and proximal to mid left anterior descending arteries. There was a more diffuse lesion in the small mid circumflex artery. Stress testing was consistent with a high risk of future events and the patient was symptomatic, despite medical therapy, necessitating revascularization. The angiographic complexity of the coronary arteries’ stenoses was limited, suggesting a successful outcome with percutaneous revascularization. A 3.0 × 20 mm drug-eluting stent was deployed in the left anterior descending artery, with excellent angiographic result. Subsequently, a 3.0 × 12 mm drug eluting stent was deployed in the right coronary artery, also with excellent result. Given the limited distribution of the left circumflex artery, and the absence of detectable ischemia in that distribution, revascularization of this artery was deferred. The patient had an uncomplicated clinical course and was discharged with dual antiplatelet therapy for 1 year.
Commentary: This case was selected to demonstrate clinical decision-making in stable coronary artery disease. The patient’s left ventricular compromise and ongoing symptoms despite medical therapy were the indications for revascularization. While the patient had multivessel coronary artery disease, the angiographic complexity was limited. This suggested a favorable outcome with a percutaneous approach utilizing drug-eluting stents.
ACUTE CORONARY SYNDROMES ST SEGMENT ELEVATION MYOCARDIAL INFARCTION
Indications for Angiography and Percutaneous Coronary Intervention
It has been estimated that annually 610,000 Americans will have a new myocardial infarction (MI) and 325,000 will have a recurrent MI.3 While the incidence of ST segment elevation myocardial infarction (STEMI) has been declining, the incidence of non-ST segment elevation myocardial infarction (NSTEMI) has increased.20 It has been suggested that the increase in the incidence of NSTEMI might be related to improved detection with the use of more sensitive biomarkers. Over the past six decades, the annual death rate for coronary artery disease has declined progressively,3,21 and it is today >50% lower than it was in 1950. This reduction is owing to a combination of factors including the institution of ICU care and EMS services, the decline in the rate of STEMI,3,20 improved primary and secondary prevention through GDMT20 and, more recently, by further evolution of reperfusion therapy for STEMI. Reperfusion therapy, by which coronary blood flow is reestablished through pharmacologic (thrombolytic) or mechanical (primary PCI) means, is the hallmark of therapy for STEMI. Primary PCI, when available in a timely fashion, is more effective than thrombolytic therapy for the treatment of STEMI (see Chapter 30), and it is associated with a significant reduction in mortality, reinfarction, and stroke. Despite these differences, the key to STEMI management depends on the timely establishment of reperfusion. Current ACC/AHA Guidelines place primary PCI as a Class I indication, when performed within 12 hours of symptom onset, when it can be performed in a timely fashion (goal within 90 minutes of medical contact), in patients ineligible for thrombolytic therapy, and in patients presenting with heart failure and/or cardiogenic shock.8,22 Thrombolytic therapy remains a viable and Class I indication for those patients who are unable to receive primary PCI within 120 minutes from first medical contact (FMC).22
Technical Considerations
Angiography and PCI should be performed expeditiously, with the goal to minimize the time to successful reperfusion. To this end, as described in Chapter 30, most operators routinely perform a diagnostic angiography of the “nonculprit” vessel initially, based upon ECG localization, and then perform angiography of the culprit vessel with a guide catheter. In vessels in which thrombotic obstruction persists, initial wiring attempts with a soft, hydrophobic wire are advisable, as most lesions are soft and easily crossed. Upon crossing the lesion, confirmation of intraluminal position, either based on angiography or in the event of persistent occlusion, by Dottering with a balloon to allow some distal flow, is advised. In patients with persistent obstruction, the balloon may be advanced distal to the obstruction and the wire removed, and careful, manual injection of contrast through the wire lumen can confirm intraluminal position. Thereafter balloon inflation of the thrombotic occlusion can proceed. In patients with large visible thrombi, or proximal occlusions, many operators will proceed initially with aspiration thrombectomy. The TAPAS trial demonstrated an acute improvement in coronary blood flow and a reduced incidence at 1 year of cardiac death and the composite of death and nonfatal reinfarction with aspiration thrombectomy.23 This approach carries a level IIa indication in current guidelines.22 Stent implantation can then follow. Both bare-metal and drug-eluting stents have been shown to be effective. Decisions about stent type remain operator dependent and should be based on vessel size and other angiographic factors, as well as clinical variables, including likelihood of patient compliance with dual antiplatelet therapy. In all cases, proper vessel sizing is critical in order to ensure adequate stent expansion and strut apposition, thereby reducing the risk of stent thrombosis.
STEMI often occurs in patients with multivessel CAD, with significant lesions in “non-culprit” vessels. Current guidelines argue against immediate treatment of “non culprit” lesions at the time of primary PCI8 (Class III indication). The guidelines are supported by several registry analyses and randomized clinical trials, as well as by a recent large meta-analysis showing that in the setting of primary PCI for acute myocardial infarction, staged PCI is associated with lower short- and long-term mortality when compared with simultaneous culprit vessel PCI and multivessel PCI.24
CASE 41.2
A 45-year-old man with no prior cardiac history and risk factors limited to cigarette smoking presented to a rural hospital emergency room with 3 hours of worsening substernal chest discomfort. Initial EKG was consistent with acute anterior wall myocardial infarction (Figure 41.2). The patient was administered aspirin, prasugrel, and unfractionated heparin per protocol.
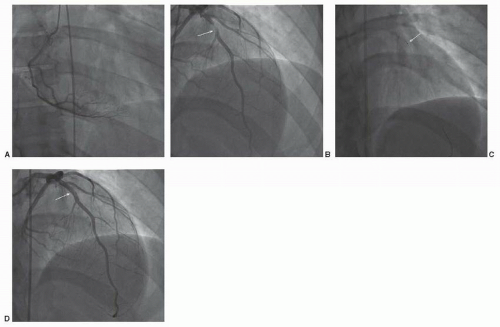
Expedited transfer to a nearby primary PCI center was arranged with a transport time of 20 minutes. Coronary angiography was performed (Figure 41.3). This demonstrated a culprit lesion in the mid segment of the left anterior descending artery, highly suggestive of a large intraluminal thrombus. The lesion was crossed with a soft wire and aspiration thrombectomy was performed with evident thrombus aspiration (Figure 41.4). Subsequently a drug-eluting stent was deployed in “direct” fashion.
The patient had a stable postprocedural course. Medical therapy at discharge included indefinite aspirin therapy, prasugrel for 12 months, an ACE inhibitor, and a beta blocker. Smoking cessation was initiated in the hospital and continued in cardiac rehabilitation.
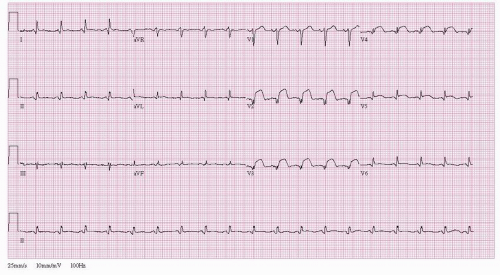 Figure 41.2 ST segment elevation myocardial infarction. Twelve-lead electrocardiogram demonstrating ST segment elevation leads V2-V6. |
Commentary: This case was selected to demonstrate the importance of rapid reperfusion therapy in management of ST segment elevation myocardial infarction. Primary PCI was selected given its rapid availability owing to a coordinated system of care in this rural region.22 Had this been lacking, or had transport time to a PCI-capable facility been longer, initial treatment with thrombolytic therapy would have been appropriate, as would have been had the patient’s presentation been closer to symptom onset, despite the availability of PCI. The significant thrombus burden favored the initial employment of aspiration thrombectomy prior to stent deployment.
NON-ST SEGMENT ELEVATION ACUTE CORONARY SYNDROME
Indications for Angiography and Percutaneous Coronary Intervention
Coronary angiography, with an intent of revascularization (surgical or percutaneous), is a Class I recommendation for patients presenting with non-ST segment elevation acute coronary syndrome, unstable angina, or myocardial infarction. Patients with refractory ischemia—including angina, or hemodynamic or electrical instability—or more stable patients at higher risk for future clinical events should undergo early angiography, and if indicated PCI.8 Large randomized clinical trials utilizing a background of contemporary antithrombic therapy demonstrated that an initial strategy of angiography followed by appropriate revascularization reduced the incidence of death and recurrent myocardial infarction, as compared to a more conservative initial approach of medical therapy and noninvasive risk stratification.25, 26, 27 Early angiography and subsequent revascularization (6 to 24 hours), as compared to “cooling off,” with later angiography and revascularization, reduce clinical events (composite of death, myocardial infarction, or CVA in high-risk Acute Coronary Syndrome (ACS) patients).
In ACS patients undergoing coronary angiography, the determination of revascularization strategy (PCI versus CABG) should be similar to that for patients with stable CAD. The patient’s angiographic profile, likelihood of success, clinical variables, and patient preference should all be considered.
Management
For patients with non-ST elevation ACS, appropriate medical management including aspirin, ADP receptor blockers, and anticoagulation with either unfractionated or low molecular weight heparin is mandatory (see Chapter 5). Additional
medical therapy including beta-blockers and blood pressure control in conjunction with aggressive lipid lowering therapy with statins is also indicated.28,29
medical therapy including beta-blockers and blood pressure control in conjunction with aggressive lipid lowering therapy with statins is also indicated.28,29
Approaches to PCI should be based on anatomic and clinical factors. Both bare-metal and drug-eluting stents can be utilized. As in the setting of STEMI, stent choice should be predicated on risk of restenosis, stent thrombosis, patient compliance, and other technical and clinical considerations. With fourth-generation drug-eluting stents being widely employed, stent delivery is rarely a consideration in determining if a bare or coated stent is to be employed. In patients with multivessel CAD undergoing PCI, multivessel intervention in a single setting is commonplace. That said, consideration of contrast burden and risk of contrast-induced nephropathy (CIN), radiation dose, initial lesion result, the extent of myocardium at risk, and other patient-specific factors should guide whether staging of secondary lesions should be considered.
CASE 41.3
An 82-year-old man with a history of coronary artery disease presented with severe chest pain and diaphoresis. Electrocardiography demonstrated dynamic anterolateral T-wave inversions, and cardiac markers (troponin) were borderline. The patient was stabilized with aspirin, unfractionated heparin, beta blocker, and nitrates. The patient had undergone coronary angiography and placement
of bare-metal stents in the proximal and middle left anterior descending artery 2 years earlier. Shortly after stent placement the patient developed recurrent episodes of both gastrointestinal and genitourinary bleeding requiring transfusion. Clopidogrel had been stopped at that time and was not restarted during the current admission. Prior to angiography the patient expressed that he was adamantly opposed to coronary artery bypass surgery owing to the need for prolonged recovery and risk of stroke, both of which would prevent his wife from living independently.
of bare-metal stents in the proximal and middle left anterior descending artery 2 years earlier. Shortly after stent placement the patient developed recurrent episodes of both gastrointestinal and genitourinary bleeding requiring transfusion. Clopidogrel had been stopped at that time and was not restarted during the current admission. Prior to angiography the patient expressed that he was adamantly opposed to coronary artery bypass surgery owing to the need for prolonged recovery and risk of stroke, both of which would prevent his wife from living independently.
The patient underwent coronary angiography (Figure 41.5), which demonstrated a culprit lesion in the middle left anterior descending artery at the site of the earlier stent. In addition, there were high-grade stenosis in the right and circumflex arteries. The limited angiographic complexity (low SYNTAX score) and preserved systolic function, suggested that PCI would afford a good outcome and meet the patient’s desire to avoid surgery. However, the patient’s poor candidacy for long-term dual antiplatelet therapy precluded multiple drug-eluting stents. Fractional flow reserve was performed on the right coronary (FFR = 0.84) and circumflex (FFR = 0.91) arteries. Accordingly, revascularization of these lesions was deferred. Given the discreet segmental nature of the in-stent restenosis of the middle left anterior descending lesion and the patient’s bleeding risk, conventional balloon angioplasty with a 2.5 × 12 mm balloon was
performed. This resulted in an excellent angiographic result. The patient was discharged with a limited course of dual antiplatelet therapy and optimal medical therapy for his residual coronary disease. He had an excellent long-term outcome.
performed. This resulted in an excellent angiographic result. The patient was discharged with a limited course of dual antiplatelet therapy and optimal medical therapy for his residual coronary disease. He had an excellent long-term outcome.
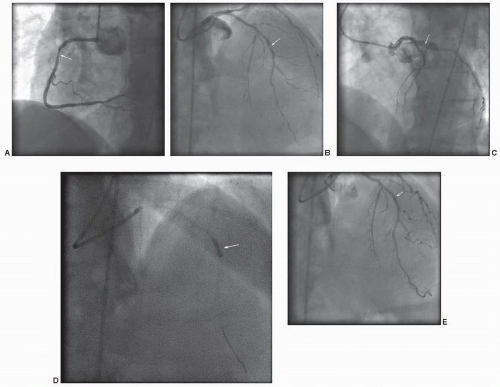 Figure 41.5 Non-ST segment elevation acute coronary syndrome. A. Angiography of the right coronary artery in the left anterior oblique angle demonstrates a severe stenosis of the proximal segment (arrow). B. Right anterior oblique view with cranial angulation demonstrates severe stenosis of the mid left anterior descending artery, at the site of a previous stent (arrow). C. Left anterior oblique view with cranial angulation demonstrates moderate to severe stenosis of the proximal circumflex artery (arrow). D. Balloon angioplasty of mid left anterior descending in-stent restenosis with a 2.5 × 12 mm balloon (arrow). E. Excellent angiographic result of mid left anterior descending artery in-stent restenosis (arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|