Profiles in Congenital Heart Disease
Gabriele Egidy Assenza
Robert Sommer
Michael J. Landzberg
Congenital heart disease has become more common not only in the pediatric but also in the adult catheterization laboratory, owing to increasing survival of patients with lesions surgically corrected in childhood and to the increasing availability of catheter-based treatments for lesions that do not present until adulthood. Some basic issues regarding catheterization in such patients have been reviewed in Chapter 9, and some of the interventional techniques have been reviewed in Chapter 35. This chapter presents a series of real-world profiles illustrating some of these basic principles.
ADULT WITH PULMONIC STENOSIS
CASE STUDIES
CASE 45.1
A 72-year-old woman presented with a loud murmur since birth and several years of progressive shortness of breath. Pulse oximetry showed an arterial oxygen saturation of 90% to 91% at baseline, falling to mid-80s during a stress test. Transthoracic echo demonstrated valvar pulmonary stenosis with a peak instantaneous gradient of approximately 115 mmHg. A bubble contrast injection was positive for a right-to-left shunt at the atrial level, consistent with a diagnosis of patent foramen ovale (PFO).
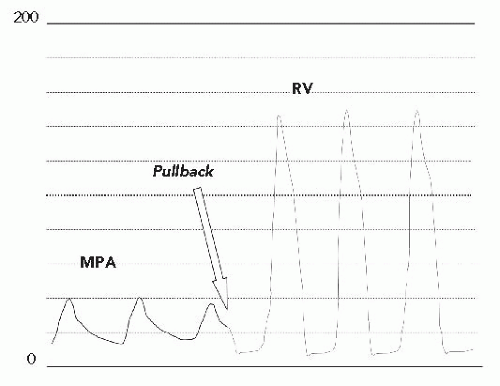
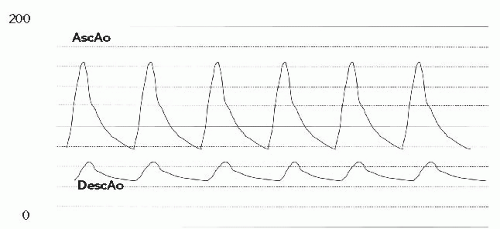
In the cath lab under intravenous sedation, an 8F sheath was placed in the femoral vein and a 5F sheath was placed in the femoral artery. A multipurpose catheter was advanced from the femoral vein to obtain right-side pressures and saturations and to cross the PFO to obtain leftside data. Oxygen saturation in the pulmonary veins was 96% (room air) with a simultaneous aortic saturation of 89%, defining a right-to-left shunt. Right atrial (RA) and left atrial (LA) filling pressures were elevated (RA mean, 15 mmHg; LA mean, 12 mmHg) with the mean RA pressure being 2 to 3 mmHg higher than the mean LA pressure. There was severe right ventricular (RV) hypertension, consistent with the echocardiographic gradient (RV pressure, 130/15), with mildly elevated pulmonary artery (PA) pressure (Figure 45.1). There were no additional obstructions at the branch PAs. There was no mitral stenosis, obstruction of the left ventricular outflow, or aortic arch. Coronary angiography showed no obstructive disease.
A Berman catheter was placed in the right ventricle and a right ventricular angiogram was obtained. The ventricle was severely hypertrophied with preserved systolic function. The pulmonary valve was thin and doming, with a jet of contrast seen through the small orifice of the doming valve (Figure 45.2). There was post-stenotic dilatation of the main pulmonary artery (MPA) and the left pulmonary
artery (LPA), with the right pulmonary artery (RPA) dilated to a lesser extent. (This is typical as the high-velocity flow is directed into the LPA: Figure 45.3.) During systole, there was dynamic subvalvar muscular (infundibular) narrowing of the outflow tract.
artery (LPA), with the right pulmonary artery (RPA) dilated to a lesser extent. (This is typical as the high-velocity flow is directed into the LPA: Figure 45.3.) During systole, there was dynamic subvalvar muscular (infundibular) narrowing of the outflow tract.
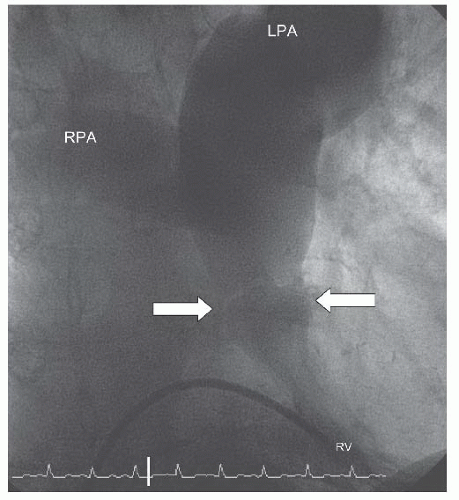
 Figure 45.2 Doming pulmonary valve leaflets in early systole and the jet of contrast emerging into the MPA from the stenotic valve orifice (arrows). MPA, main pulmonary artery; RV, right ventricle. |
The pulmonary valve was dilated with the Inoue balloon technique—techniques of balloon valvular dilation vary, with some operators preferring the relative ease and simplicity of the Inoue balloon methodology, while others prefer a greater sense of waist and compliance assessment that can be afforded by standard single- and double-balloon dilation (see Chapters 33 and 40). An Inoue balloon was selected with a maximum inflation diameter a few millimeters larger than the measured diameter of the valve annulus, but was prepared with only enough volume to expand to the size of the annulus. A stiff exchange-length 0.032-inch guidewire was advanced through a multipurpose catheter into the distal LPA. The Inoue balloon was straightened and introduced through a 14F sheath at the femoral vein. The balloon was advanced over the wire to the RA, where the straightening rod was removed to ease passage through the two valves. (In some cases, manipulation through the tricuspid valve and the RV outflow tract can be facilitated by partial inflation of the balloon.) Once in the MPA, the distal portion of the balloon was inflated and pulled back against the valve tissue. Then the proximal portion and middle waist were inflated. There was a pop as the indentation of the valve on the balloon was eliminated. The stiff guide wire provided
with the Inoue was advanced through the balloon to the LPA. A side-arm valve was added to the back of the catheter, and a pressure transducer was attached to the side port of the valve. The balloon was then withdrawn, over the wire, back into the RV to assess the residual gradient.
with the Inoue was advanced through the balloon to the LPA. A side-arm valve was added to the back of the catheter, and a pressure transducer was attached to the side port of the valve. The balloon was then withdrawn, over the wire, back into the RV to assess the residual gradient.
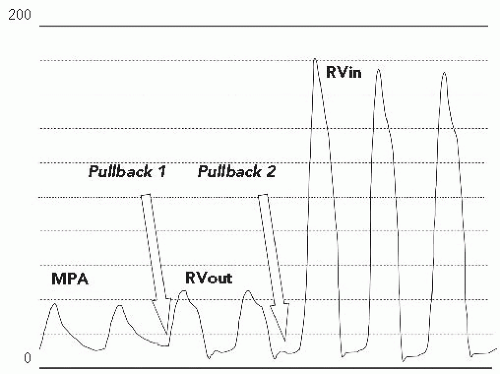
On pullback, an increase in the gradient across the RV outflow tract was noted. However, closer inspection of the pullback tracing revealed that the gradient had shifted from the level of the valve to the subvalvar (infundibular) area (Figure 45.4). On pullback 1, from the MPA into the RV, the diastolic tracing confirmed that the catheter was in a ventricular chamber, with virtually no systolic gradient across the valve. Pullback 2, identified that the systolic gradient was located within the ventricular chamber, at the subvalvar level. Arterial saturations fell acutely into the mid-80s following valvuloplasty. A repeat angiogram revealed markedly improved mobility of the valve leaflets with a larger valve orifice (Figure 45.5). However, there was profound dynamic obstruction at the level of the valve (Figure 45.6). The patient was hydrated intravenously and started on an intravenous beta-blocker infusion. Over 10 to 15 minutes, systemic arterial oxygen saturations rose to the mid-90s (%), as the gradient fell; over the subsequent 6 weeks, the peak instantaneous gradient by Doppler decreased even further, systemic arterial saturations normalized, and oral beta-blocker therapy was discontinued.
Discussion
Valvar pulmonary stenosis (PS) is far less common in the adult population than in children and young adults, but when left untreated into adulthood, it can cause significant symptoms. Unlike the aortic valve, the pulmonary valve does not
generally calcify and is thus typically amenable to balloon dilatation. The long-term follow-up of repaired valvar PS, by surgical or balloon valvuloplasty, demonstrates that in most cases it is a highly successful, if not curative, procedure.1 Late valvar insufficiency may also cause symptoms of shortness of breath, or fatigue, on exertion, but is more frequently a product of surgical intervention.2
generally calcify and is thus typically amenable to balloon dilatation. The long-term follow-up of repaired valvar PS, by surgical or balloon valvuloplasty, demonstrates that in most cases it is a highly successful, if not curative, procedure.1 Late valvar insufficiency may also cause symptoms of shortness of breath, or fatigue, on exertion, but is more frequently a product of surgical intervention.2
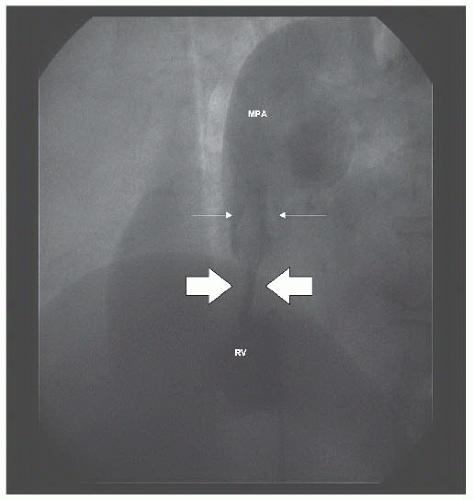
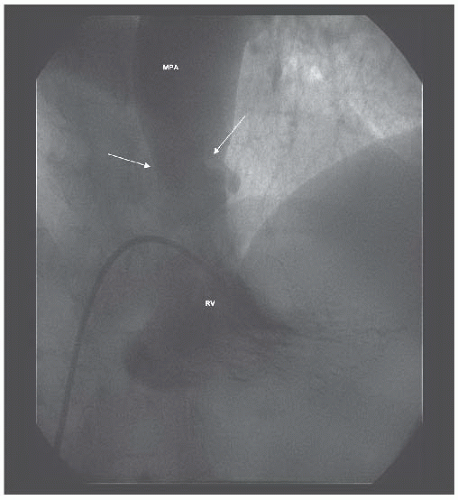 Figure 45.5 Marked improvement in valvar excursion after balloon dilation (compare with Figure 45.2). White arrows denote tips of valve leaflets. MPA, main pulmonary artery; RV, right ventricle. |
Severe infundibular stenosis after valvuloplasty, which has been termed the “suicide” right ventricle3 in surgical series, is physiologically identical to left ventricular outflow tract obstruction in hypertrophic cardiomyopathy; hypovolemia and increased inotropic states augment the degree of obstruction, which is typically a product of long-standing PS with RV hypertrophy. The cyanosis in this patient was driven by long-standing RV hypertrophy and reduced RV diastolic compliance sufficient to elevate RA pressures and resistance to filling, contributing to preferential right-to-left shunt across the patent foramen ovale.
In children, in whom most PS repairs are performed, standard valvuloplasty balloons are very effective, and also inexpensive. In adults and in teenagers, the size of the valve annulus is such that a double-balloon technique is often required to obtain enough dilating force and diameter. The Inoue balloon (see earlier discussion) can be selected to suit larger diameters and is variable in its inflation size, so that a larger inflation size can be used without needing to change the catheter.4
Balloon valvuloplasty is a highly effective and extremely safe catheter intervention. It should be considered the procedure of choice for valvar PS.
COARCTATION OF THE AORTA
CASE 45.2
A 32-year-old man presented with poorly controlled hypertension and bilateral lower extremity claudication. He had been prescribed, and was compliant with, beta-blocker and ACE inhibition therapy, with continued poor blood pressure control and slightly impaired renal function. Blood pressure in the right arm was 165/90, with no palpable femoral pulses and a blood pressure of 75/40 in the lower extremities. There was an apical click, a soft systolic murmur, and a diastolic decrescendo murmur. Cardiac magnetic resonance (CMR) revealed a discrete coarctation of the aorta approximately 2 cm distal to the left subclavian artery.
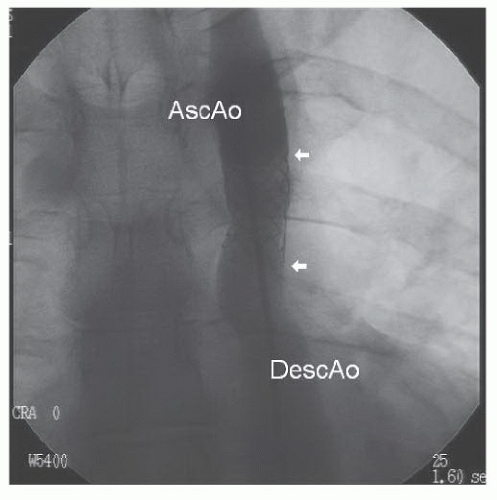
Under intravenous propofol sedation, sheaths were placed in the femoral artery and vein. Right-side hemodynamics were obtained. A pigtail catheter advanced from the femoral artery would not pass around the aortic arch. A floppy-tipped wire was then manipulated through a multipurpose catheter around the aortic arch, after which the pigtail catheter was re-advanced over the wire and an ascending angiogram was obtained (Figure 45.7). With a proximal aortic isthmus diameter of 18 mm and a distal descending aortic measurement of approximately 21 mm, the minimum dimension at the coarctation site was approximately 2.5 mm. The angiogram demonstrated filling of the descending aorta from intercostal and internal mammary collaterals.
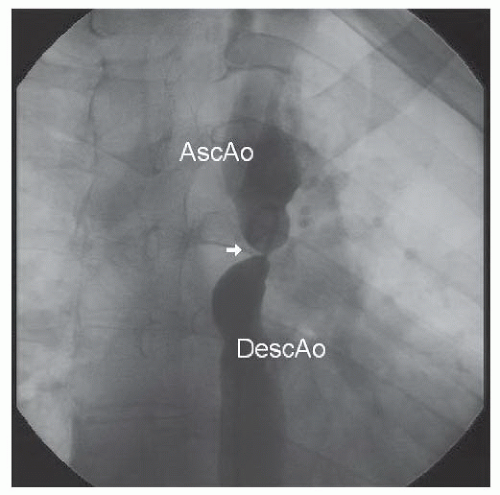 Figure 45.7 Discrete, severe coarctation of the aorta (white arrow). AscAo, ascending aorta; DescAo, descending aorta. |
A stiff wire with a hand-formed loop was advanced through the pigtail catheter around the arch to the ascending aorta, and the pigtail catheter was removed. A 10F Mullins sheath (through which the pigtail catheter was replaced to the ascending aorta) was advanced over the wire to the descending aorta (Figure 45.8). The pigtail catheter was removed, and the long sheath was advanced through the coarctation to the ascending aorta. At the treating center, predilation of the coarctation site, to allow assessment of aortic compliance, was not performed. A balloon-dilatable Palmaz iliac stent (Johnson & Johnson) was mounted on a 10-mm balloon catheter and advanced through the sheath to the level of the coarctation site. The sheath was withdrawn into the descending aorta, and an angiographic injection was performed through the side port of the Mullins sheath to help position the stent. The balloon was inflated rapidly, expanding the stent to 10 mm (Figures 45.9 and 45.10).
The pigtail catheter was reinserted, and repeat measurement of simultaneous pressures was carried out (Figure 45.11). There was a small residual gradient across the stent, but the ascending aortic pressure was markedly reduced, and the descending aortic pressure increased to the normal range. Because of the small initial diameter of the coarctation segment, we elected to do full dilation in two stages. The sheaths were removed, and hemostasis was obtained. Six months later, the patient returned with a small
persistent gradient, though with improved blood pressure (BP) control and elimination of the symptoms in his lower extremities. From a femoral access, a 15-mm and then an 18-mm balloon (Figures 45.12 and 45.13) were used sequentially to redilate the stent to equal the size of the proximal aorta, thus eliminating the residual pressure gradient.
persistent gradient, though with improved blood pressure (BP) control and elimination of the symptoms in his lower extremities. From a femoral access, a 15-mm and then an 18-mm balloon (Figures 45.12 and 45.13) were used sequentially to redilate the stent to equal the size of the proximal aorta, thus eliminating the residual pressure gradient.
Discussion
De novo diagnosis of coarctation in the adult population is uncommon, but patients who have had previous aortic coarctation surgery may have residual obstruction at surgical sites. Any patient with systemic arterial hypertension should have examination of the lower extremity pulses, and the four extremity pressures should be checked at least once during their lifetime to rule out this disease. In children who have not reached full adult size, surgery was historically the preferred therapy for native coarctation, although increasingly, centers with appropriate expertise offer primary balloon angioplasty when it is both anatomically and physiologically suitable. For recurrent coarctation after surgical repair in children, however, balloon angioplasty is accepted as the procedure of choice, when feasible. Stenting is generally not used in younger children given their growth potential, but has become widely accepted in older children, as well as in adults with coarctation.5,6 Stenting appears to provide more control in dilating the coarcted segment and eliminates the need for oversizing of the balloon with attendant risks of aortic rupture, tear, or dissection.
Many centers are increasingly performing “predilation” (balloon expansion at the site intended to ultimately receive a stent) in a staged fashion and with limitations in permitted waist-balloon diameter ratio, so as to assess compliance of the aortic wall and to presumably decrease
the risk of both underdilation and vessel rupture. In this patient’s case, predilation was not chosen by the intervention center—though dilation was performed in a staged approach—owing to the small size of the native vessel. The initial stent size was limited to 4 to 5 times the initial diameter of the coarctation lesion; the patient returned after 6 months (with presumptive healing of the stent site) for fuller stent expansion.
the risk of both underdilation and vessel rupture. In this patient’s case, predilation was not chosen by the intervention center—though dilation was performed in a staged approach—owing to the small size of the native vessel. The initial stent size was limited to 4 to 5 times the initial diameter of the coarctation lesion; the patient returned after 6 months (with presumptive healing of the stent site) for fuller stent expansion.
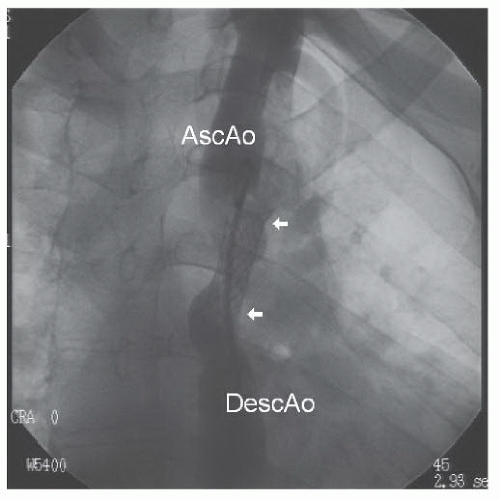 Figure 45.10 Repeat angiogram after initial stent inflation. AscAo, ascending aorta; DescAo, descending aorta. White arrowheads mark extent of the stent. |
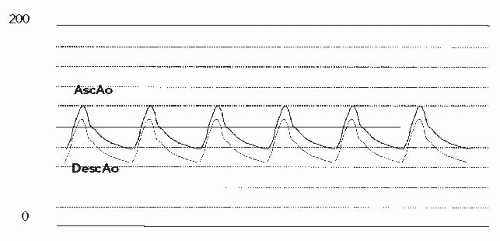 Figure 45.11 Simultaneous pressure tracings from ascending aorta (AscAo) and descending aorta (DescAo), post-initial stent implant. |
Stent placement is also possible in more proximal lesions, such as coarctation that involves the transverse arch and isthmus, and may impinge on the left subclavian or even the carotid vessels. Lesions in each of these locations have been successfully treated with stent angioplasty without adverse neurologic events or arm ischemia.7 Some congenital interventional cardiologists have endorsed the use of covered stents to reduce the risk of acute aortic injury. These stents have been utilized as “rescue” treatment in patients with periprocedural dissection, rupture, or aneurysm formation, and have been suggested for use (a) for postprocedural (surgery or percutaneous) aneurysms located at or near the coarctation site, (b) in patients in need of repair with native or repaired coarctation, who have particular risk of procedural complication (e.g., anatomy with atretic/near atretic coarctation, tortuosity, and long segmental involvement), and (c) for stent failure (stent fracture or in-stent restenosis). Anecdotal data support use of covered stents under specific local and FDA protocol for periprocedural dissection or rupture. For any other indication, it should be noted that covered stents do not have FDA approval for treatment of aortic coarctation in the United States. They have been utilized as implantation therapy mainly in Europe, but very scarce data are available on the longer-term follow-up in patients who have undergone
this treatment. The ongoing Coarctation of the Aorta Stent Trial (COAST) is a nonrandomized, multicenter, open-label study addressing the safety and efficacy profile of covered Cheatham Platinum stent use in children, adolescents, and adults with coarctation of the aorta and estimated or measured transcoarctation pressure gradient of at least 20 mmHg. This study will be useful to better define the clinical indications and efficacy of this type of stent in percutaneous treatment of coarctation of the aorta.
this treatment. The ongoing Coarctation of the Aorta Stent Trial (COAST) is a nonrandomized, multicenter, open-label study addressing the safety and efficacy profile of covered Cheatham Platinum stent use in children, adolescents, and adults with coarctation of the aorta and estimated or measured transcoarctation pressure gradient of at least 20 mmHg. This study will be useful to better define the clinical indications and efficacy of this type of stent in percutaneous treatment of coarctation of the aorta.
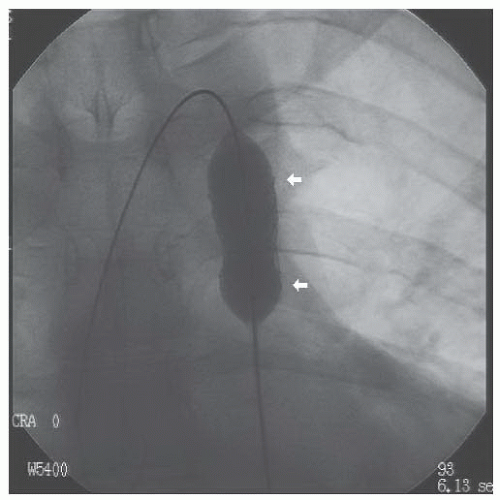 Figure 45.12 Subsequent inflation of stent at follow-up cath. White arrowheads mark extent of the stent. |
ATRIAL SEPTAL DEFECT
CASE 45.3
A 43-year-old woman with no past medical history presented with increasing shortness of breath on exertion. Oxygen saturation was normal by pulse oximetry. An echocardiogram revealed a dilated right atrium and right ventricle with a tricuspid regurgitation velocity of approximately 3.8 cm/second; transesophageal echo revealed a 1.7-cm atrial septal defect with predominantly left-to-right shunting (Figure 45.14).
The patient was taken to the cardiac catheterization lab, and two sheaths were placed in the right femoral vein under local anesthesia and conscious sedation. Through the first, an intracardiac echo (ICE) catheter (Acuson, Siemens) was passed to the right atrium for imaging the septum. Through the second sheath, a multipurpose catheter was inserted to perform hemodynamic measurements. There was only moderate pulmonary hypertension (PA pressure of 68/14 mmHg with simultaneous aortic pressure of 140/78 mmHg). The calculated 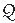 p/
p/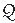 s ratio was 2.3:1 with an indexed pulmonary vascular resistance of 3.2 Units (see Chapter 12).
s ratio was 2.3:1 with an indexed pulmonary vascular resistance of 3.2 Units (see Chapter 12).
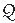 p/
p/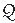 s ratio was 2.3:1 with an indexed pulmonary vascular resistance of 3.2 Units (see Chapter 12).
s ratio was 2.3:1 with an indexed pulmonary vascular resistance of 3.2 Units (see Chapter 12).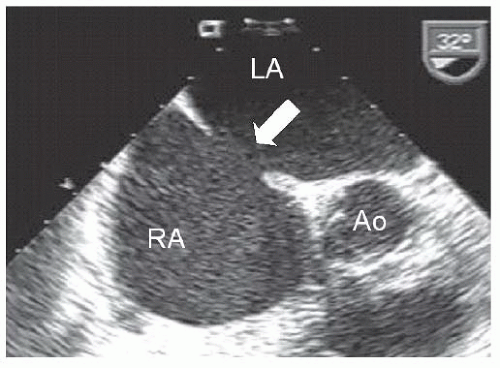 Figure 45.14 Transesophageal image of secundum atrial septal defect (white arrow). RA, right atrium; LA, left atrium; Ao, aorta. |
The multipurpose catheter was then used to cross the atrial septal defect and was manipulated to the left upper pulmonary vein. A stiff guidewire was passed into the pulmonary vein. A balloon-sizing catheter was advanced over the wire to straddle the defect and was inflated until flow stopped by ICE (Figure 45.15). At that point, the diameter of the waist on the balloon catheter was 19 mm. Circumferential septal rims were adequate (as assessed by echo), and a 20-mm Amplatzer Septal Occluder (AGA Medical, Minneapolis, MN) was selected (Figure 45.16). The balloon catheter was removed, and a 9F Mullins sheath was advanced over the wire and into the left atrium. The Amplatzer device was collapsed and advanced through the delivery sheath until the left atrial side of the occluder opened in the left atrium and was pulled back toward the septum (Figure 45.17). When the left atrial disc of the occluder was adjacent to the septum, the middle waist and right atrial side were opened, and the device was allowed to return to its native shape. In this case, because of the relatively deficient retroaortic rim (current FDA regulations recommend avoidance of device implantation in such circumstances), the device appears splayed out somewhat over the aortic root (Figure 45.18), but is in position with no residual shunt. The device was released without difficulty, sheaths were removed, and the patient was discharged 4 hours later.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree