(1)
The Lindner Center for Research and Education The Christ Hospital, The Christ Hospital Physicians Ohio Heart and Vascular Center, Cincinnati, OH, USA
Keywords
Data acquisitionData reconstructionImage displayData acquisition systemDASPatient selectionImaging the morbidly obeseIodine fluxSignal to noise ratioContrast administration rateCoronary calcificationBlooming effectPartial volume averagingECG editingBypass graftsCoronary stentsPatient preparationB blockerBeta blockerMetforminContrast dye allergyIVIntravenousElectrocardiogram lead placementNitroglycerinSublingual nitroglycerinCCTA protocolsScan timingTriggering scan modeProspective imagingRetrospective imagingRadiation sparing protocolsTube potentialTube currentX ray fluxECG triggeringDose modulationECG based dose modulationAnatomy based tube current adaptationRadiation dosePitchScan protocolAcquisition field of viewaFOVNoise-reducing reconstruction algorithmsIterative reconstructionScan timingTest bolusBolus trackingScan fieldScan field directionContrastContrast injection techniquesPhase selectionPaddingMultiphasic analysisReconstruction filtersConvolution filterKernalHounsfield UnitH.UPartial scanMultisegmental reconstructionTriggeringAtrial fibrillationImage displayComputer workstationVolume rendered techniqueVRTMultiplanar reformatMPRMaximum intensity projectionMIPCurved multiplanar reformatcMPRCurved maximum intensity projectioncMIPSingle plane multiplanar reconstructionGeneral
Technological advances in Computed tomographic (CT) imaging have made possible the routine and reliable use of cardiac computed tomographic angiography (CCTA) to image the heart and coronary arteries. Improvements in detector characteristics have increased the x and y axis spatial resolution of a CT scanner to the neighborhood of 0.4 mm which is necessary to successfully image the coronary arteries. Further, advances in CT collimation have increased the z axis spatial resolution to match that of the x and y axes and have made possible the formation of isotropic voxels which are necessary to properly manipulate the CCTA data in all planes without distortion. Improvement in gantry mechanics and rotation time and the advent of the dual source scanner have increased the temporal resolution of CCTA to allow the scanner to effectively freeze the heart and its arteries for imaging. In addition, advances in the ability to quickly start and stop the gantry and table alike along with improvements in ECG gating and tracking have permitted the routine use of prospective imaging in CCTA, which has significantly reduced radiation exposure. Finally, increasing numbers of detector rows have increased the coverage of the heart obtained in one gantry rotation allowing the heart to be imaged in one breath hold, further reducing data reconstruction artifacts. See Fig. 2.5.
Data Acquistion
Three general steps are required to successfully create a CCTA image. These are data acquisition, data reconstruction and image display (Fig. 3.1). Data acquisition involves collecting the raw data when imaging the patient. Data reconstruction describes the process of reconstructing the data into a displayable and relevant format for the reader. Image display requires the reformatting of the image on the computer workstation to allow proper interpretation of the images.
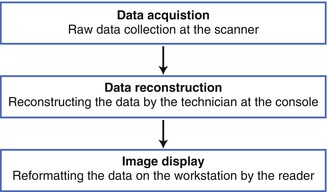

Figure 3.1
A depiction of the three major steps to performing a cardiac computed tomographic angiogram
Data acquisition is the stage when the raw data are collected by the scanner as the patient is being scanned. The x rays originate from the x ray source and are observed by the detectors, which then transfer the data to the data acquisition system (DAS) where they are stored in a mathematical format in a computerized bin as raw axial data to be reconstructed for display. Data acquisition involves scanning portions of the heart with each gantry rotation to create image slabs. As depicted in Fig. 2.5, slab thickness depends on the number of detector rows and the size of each detector. Slabs are aligned and combined to form the total image (Fig. 3.2). Meaningful data acquisition involves proper patient selection, patient preparation and scan timing as well as the selection of appropriate imaging protocols and contrast injection techniques.
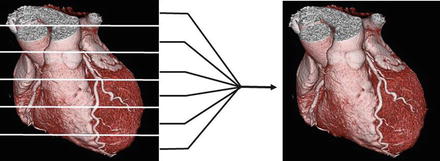

Figure 3.2
A cartoon depiction of the image formation from the addition of all of the individual slabs
Appropriate patient selection and preparation are the critical pre imaging determinants of image quality and diagnostic utility. Key aspects of adequate CCTA imaging include effective heart rate control, proper timing of the scan relative to contrast initiation, and minimizing patient movement. Post collection image reconstruction techniques and processes are also crucial to creating adequate CCTA images but cannot compensate for problems in the collection of the raw data.
Patient Selection
Appropriate patient selection and preparation are the predominate determinants of CCTA image quality and diagnostic utility. Image quality is markedly improved by achieving a slow, regular heart rate for imaging, avoiding CCTA in the very obese and by selecting patients who are able to understand and comply with directions and who are able to properly perform a breath hold. In addition, avoiding CCTA in patients known to have excessive coronary calcium will make the interpretation simpler and more accurate. All of these factors can be ascertained well in advance of the procedure except determining the amount and location of coronary calcium which is assessed on the pre-contrast calcium scoring scan.
Diagnostic imaging quality is more difficult to obtain in the morbidly obese due to attenuation artifact and reduced visualization of contrast in the coronary tree. As a general rule, CCTA is not optimal in patients whose body mass index is >40 kg/m2. However, patients should be considered on a case by case basis. For example, patients whose body mass resides predominately in the abdomen, hips and lower extremities may still undergo a diagnostic CCTA due to the absence of fat in the chest region. Additionally, for specific diagnostic questions such as evaluation of the left main or proximal coronary arteries, a diagnostic CCTA may still be obtained in the morbidly overweight.
If a large patient is imaged, steps should be taken to improve the image quality, which make a diagnostic image more likely. First, the milliampere setting should be maximized to increase the signal to noise ratio. Additionally, using thicker slices will also improve the signal to noise ratio, albeit at the expense of less z axis resolution. Maximization of the iodine flux is also necessary to increase contrast opacification. The iodine flux is related to the iodine concentration of the contrast where contrast agents with increased iodine concentration will increase coronary artery opacification. Increasing the rate of bolus administration will also improve the iodine flux. The usual rate of contrast administration is 5 ml/s. However, with larger bore (18 guage) intravenous catheters, rates as high as 6–7 ml/s may be used. It should be noted that iodine flux is also related to uncontrollable factors such as cardiac output. Patients with diminished cardiac output will have decreased iodine flux. It should be noted that low cardiac output may not be coincident with low ejection fraction.
Coronary calcification may interfere with the interpretation of a CCTA. This is due to the blooming effect of calcium, which appears, on CCTA, to be five times larger than it’s actual size. This “blooming” effect is due to partial volume averaging. This concept is illustrated in cartoon format in Fig. 3.3. Partial volume averaging is the result of the limitations of CCTA and is due to the methods of image creation. The reason for partial volume averaging is that, while excellent, the spatial resolution of CCTA is still approximately 0.4 mm. The edges of the calcium are smaller than 0.4 mm and cannot be accurately resolved by current CT scanners. Thus, the size of a voxel is larger than the size of the calcium edge. Consequently, when the calcium partially encompasses a voxel, the CT number of the entire voxel will exceed the threshold for calcium and the entire voxel will be displayed as the highest CT number despite the fact that the calcium encompasses only part of the voxel. Said another way, the resolution of the computer matrix representation of the image can be no smaller than the voxel size. The result of calcium blooming is that the coronary artery lumen may be partially or fully obscured and when this phenomenon is extensive and diffuse or even focally severe and in these instances, gradations of a particular coronary lesion may be difficult.
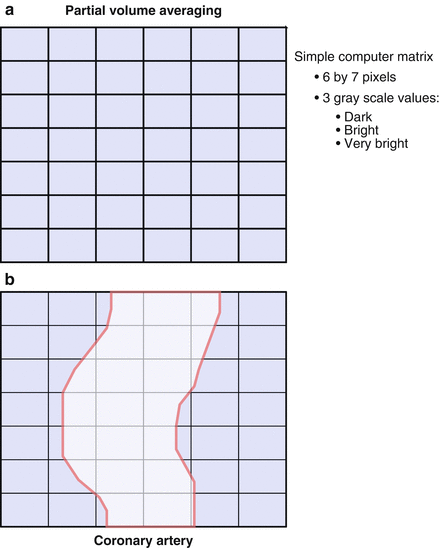
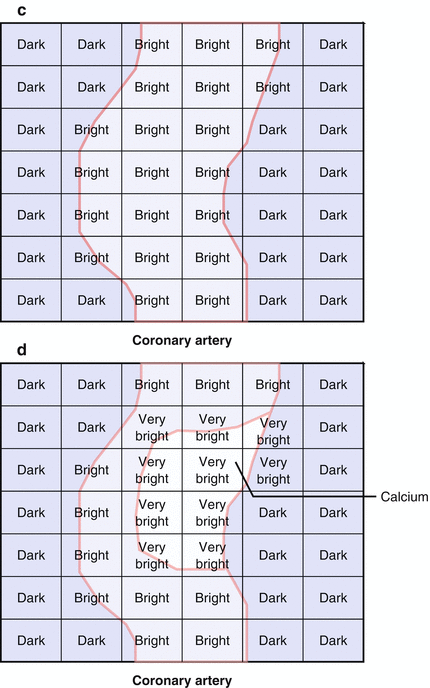
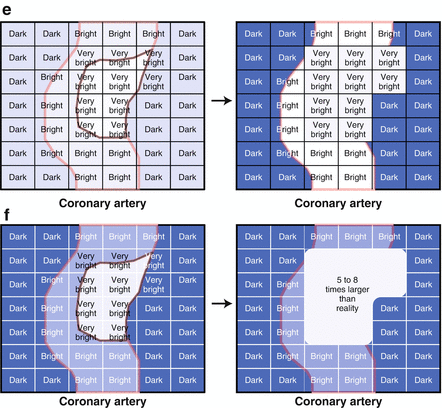



Figure 3.3
A cartoon depiction of the mechanism for calcium blooming
Methods to help resolve the issue of calcium blooming include widening the contrast window and using a sharper filter. If the calcium completely obscures the vessel lumen in all views, there is approximately a 50 % chance of an obstructive lesion in this location. If any lumen is visualized around the calcium, there is approximately a 2 % chance of an obstructive lesion [3]. How an individual reader chooses to report calcified lesions depends on the reader’s decision regarding how sensitive versus how specific he or she wishes to be. It is my opinion that sensitivity is more important and that obstructive lesions should not be missed. Therefore, my practice is to report that an obstructive lesion cannot be excluded if no lumen is visualized around the calcium and that an obstructive lesion is unlikely if lumen can be seen. There are no definitive guidelines regarding what level of coronary calcium precludes a diagnostic CCTA. Since my anecdotal experience indicates that very high calcium score scans may still be diagnostic, I do not use a calcium score threshold for cancelling a scan. Presently, there is no absolute consensus on the threshold of calcium that precludes a technically adequate and diagnostic scan [1].
Pacemakers and defibrillators also do not preclude the use of CCTA. However, it should be recognized that pacemaker and defibrillator leads may create artifacts that could obscure sections of the coronary arteries.
Regular, slow heart rhythms are mandatory for diagnostic CCTA imaging. Patients with arrhythmia during the pre-scan assessment period should, in general, not be imaged. Exceptions do exist and at times, in fact, patients in atrial fibrillation are imaged. In addition, ECG editing techniques (discussed later) can salvage scans performed during limited ectopy. Extensive ECG editing, however, will eliminate too much data for a full and diagnostic reconstruction. In general, for the evaluation of coronary arteries, only patients with slow, regular heart rhythms should be imaged.
CCTA is excellent for diagnostic imaging of bypass grafts and yields excellent diagnostic information [1]. In fact, CCTA for this purpose is an appropriate criteria for imaging [2]. However, native coronary arteries in patients who have had bypass surgery degenerate and calcify quickly and CCTA yields much less accurate information about the native coronary arteries in these patients and is an important limitation of CCTA in evaluating the patient who has had bypass surgery [1]. Additionally, the imaging of stents also pose significant challenges for CCTA. The metal in the stents create many CCTA imaging artifacts, which include but are not limited to blooming. The literature suggests that in patients with larger stents (>3.0 mm) who have a high likelihood for good image quality, whose clinical presentation suggests low to intermediate risk for in stent stenosis, CCTA can be used to rule out significant in stent restenosis [1]. However, in most instances CCTA is not the test of choice for the sole purpose of evaluating for in stent stenosis. While the lumen within larger stents (>3.0) may often be adequately visualized, CCTA solely for stent evaluation is not routinely advisable at this time. Discussion about CCTA for bypass patients and in patients who have had coronary stents placed will be discussed in detail later.
Proper patient selection accounts not only for individual patient characteristics that provide a higher likelihood for a diagnostic scan but also for appropriate patient indications that result in diagnostic utility. While data are not sufficient to justify guidelines on the topic of data driven CCTA indications, appropriate use criteria have been published and while individual judgment should be employed for each single patient, these criteria are recommended as a guide to the proper indications for CCTA [2]. For a full account of the appropriate uses of CCTA, please consult the referenced appropriate use criteria document [2]. Tables 3.1a–g categorize the current appropriate use criteria for CCTA.
(a) Detection of CAD in symptomatic patients without known heart disease |
ECG interpretable and able to exercise and intermediate probability of CAD |
ECG uninterpretable or unable to exercise and low pretest probability of CAD |
ECG uninterpretable or unable to exercise and intermediate pretest probability of CAD |
Normal ECG and cardiac biomarkers and low pretest probability of CAD |
Normal ECG and cardiac biomarkers and intermediate pretest probability of CAD |
ECG uninterpretable and low pretest probability of CAD |
ECG uninterpretable and intermediate pretest probability of CAD |
Nondiagnostic ECG or equivocal cardiac biomarkers and low pretest probability of CAD |
Nondiagnostic ECG or equivocal cardiac biomarkers and intermediate pretest probability of CAD |
(b) Detection of CAD/risk assessment in asymptomatic patients w/o known CAD |
Noncontrast CT for coronary calcium score if low risk global CHD risk estimate and family h/o premature CAD |
Noncontrast CT for coronary calcium score and intermediate global CHF risk estimate |
(c) New onset or newly diagnosed CHF and no prior CAD |
Reduced LVEF and low probability of CAD |
Reduced LVEF and intermediate probability of CAD |
(d) Preoperative coronary assessment prior to noncoronary cardiac a surgery |
Coronary evaluation before noncoronary cardiac surgery and intermediate pretest probability of CAD |
(e) Use of CCTA in the setting of prior test results |
Prior exercise testing |
Exercise testing and Duke Treadmill Score Indicating Intermediate Risk Findings |
Normal exercise test with continued symptoms |
Sequential testing after stress imaging procedures |
Discordant exercise ECG and imaging results |
Stress imaging results are equivocal |
Diagnostic impact of CaSc on whether to perform CCTA in symptomatic patients |
CaSc of <100 |
CaSc of 100–400 |
Evaluation of new or worsening symptoms in the setting of past stress imaging |
Previous stress imaging study is normal |
(f) Risk assessment post – revascularization (PCI or CABG) |
Symptomatic (ischemic equivalent) |
Evaluation of graft patency after coronary bypass surgery |
Asymptomatic |
Prior left main coronary stent with stent diameter ≥3 mm |
(g) Evaluation of cardiac structure and function |
Adult congenital heart disease |
Assessment of anomalies of coronary arterial and other thoracic arterio-venous vessels |
Assessment of complex adult congenital heart disease |
Evaluation of ventricular morphology and systolic function |
Evaluation of left ventricular function following acute MI or in CHF patients with inadequate imaging from other noninvasive methods |
Quantitative evaluation of RV function |
Assessment of right ventricular morphology in suspected arrhythmogenic right ventricular dysplasia |
Evaluation of intra and extra-cardiac structures |
Characterization of native cardiac valves in individuals with suspected clinically significant valvular dysfunction when images from other noninvasive methods are inadequate |
Characterization of prosthetic heart valves in individuals with suspected clinically significant valvular dysfunction when images from other noninvasive methods are inadequate |
Evaluation of cardiac mass (suspected tumor or thrombus) with inadequate images from other noninvasive methods |
Evaluation of pericardial anatomy |
Evaluation of pulmonary vein anatomy prior to radiofrequency ablation for atrial fibrillation |
Noninvasive coronary vein mapping prior to placement of biventricular pacemaker |
Localization of coronary bypass grafts and other retrosternal anatomy prior to reoperative chest or cardiac surgery |
Patient Preparation
Proper patient preparation both before and during the CCTA examination is essential to creating a useful CCTA image. Controlling for noncardiac motion (breathing and patient movement) as well as cardiac and coronary motion is the critical factor in preparing the patient for the CCTA examination and begins in the days prior to the patient presenting to the CT suite and continue throughout the procedure itself. Strategies to control for movement and motion involve both technology (discussed later) and pharmacology. The heart and coronary arteries move in complex patterns very rapidly through space throughout the cardiac cycle [4]. Each coronary artery moves in a different pattern and with a different velocity and degree. In fact, different segments of the same artery move differently [3]. In addition, the coronary velocity and acceleration during the cardiac cycle increase at higher heart rates [4]. See Fig. 3.4. Efforts to slow the heart rate down both before and during the procedure are critical.
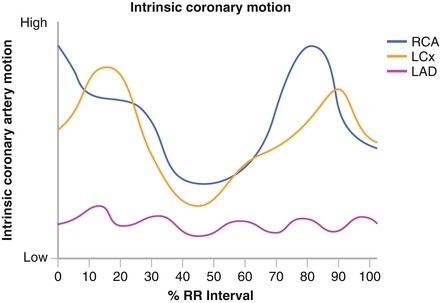

Figure 3.4
A graph depicting the movement of the coronary arteries during the cardiac cycle
Patients should present to CT suite well hydrated to avoid sinus tachycardia resulting from dehydration. Patients should be instructed to eat a light breakfast the morning of the scan. Additionally, patients should be instructed to take all scheduled medications. Missing scheduled beta blocker doses, for example, may result in withdraw tachycardia which will make the scan difficult to perform. Subjects should also be asked to avoid significant physical activity such as exercise before their scan to avoid inappropriate sympathetic activity at the time of the scan. B- blockers are most frequently used to affect heart rate control. Oral b blockers are prescribed for home use before the procedure and IV b blockers may be used at the time of the scan if needed. Pre-procedural b blockers should be prescribed based on the patient’s age, resting heart rate and whether or not the patient has ever been exposed to b blockers. We predominantly use oral (at home) and IV (just before the scan if necessary) Lopressor because we find it most affective. Calcium blockers may be used if b blockers are contraindicated as in severe reactive airway disease. As the heart rate slows, the time where the least coronary motion occurs is during isovolumic relaxation (at approximately 60–80 % of the R-R interval). Optimal right coronary images may be found at different phases than optimal left coronary images. Often the right coronary is seen best in the systolic phases. Table 3.2 illustrates the pre procedural b blocker protocol employed in our laboratory.
Table 3.2
A chart depicting one oral B blocker protocol for heart rate control prior to the scan
Heart rate (bpm) | B blocker dose (B blocker naïve patient) | B blocker dose (currently taking B blocker) |
|---|---|---|
<60 | None | None |
60–70 | Lopressor 25 mg | Lopressor 50 mg |
70–80 | Lopressor 50 mg | Lopressor 75 mg |
>80 | Lopressor 75 mg | Lopressor 100 mg |
Metformin should be avoided for 24 h before the scan and for 48 h after the scan to avoid potential adverse affects of concomitant iodinated contrast administration. Viagra, Cialis and Levitra should be avoided 48 h before the scan to eliminate the potential adverse reaction when used in combination with sublingual nitroglycerin, which is necessary for an optimal CCTA. Pre-procedural hydration as well as pre-procedural mucomyst is suggested in those with renal insufficiency. CCTA should be avoided in patients with creatinine >1.5. If an iodinated contrast allergy is present, premedication protocols should be employed.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


