Preventive Strategies for Coronary Heart Disease: Introduction
Atherosclerotic coronary heart disease (CHD), perhaps more than any other chronic condition, is ideally suited for strategies aimed at prevention (Table 51–1). CHD is extremely common and contributes to more than 1.2 million myocardial infarctions (MIs) and nearly 500,000 deaths in the United States each year.1 It is caused by numerous modifiable risk factors with high prevalence, including physical inactivity, overweight and obese states, dyslipidemia, hypertension, diabetes mellitus, and tobacco use. CHD is also associated with profound societal economic cost, with estimates of $165 billion being spent on this condition in 2009 alone.1
| • Common disease, high incidence |
| • Modifiable by behavior |
| • Long disease latency |
| • Short time between symptoms and disability |
| • Sudden death a common presentation |
| • Revascularization does not “cure” underlying disease (high residual risk) |
| • Revascularization associated with huge financial and societal cost |
Central to the treatment of CHD is the recognition that its incidence is readily modifiable by behavior. Although this is particularly true when healthy lifestyles are adopted early, there are nonetheless barriers to adoption of such behaviors. CHD is a slowly progressive disease that produces few symptoms until late into its course, thus providing ample time for prevention but limited motivation for earlier lifestyle changes. In contrast, when CHD becomes evident, there is often a short duration between symptom onset and disability, leaving less time to initiate preventive strategies. Despite vast improvements in the treatment of patients with acute CHD, such therapies are associated with tremendous cost, and affected patients remain at high risk. The consequence of the above is an unnecessarily high number of patients in the population that face increased cardiovascular (CV) risk.
The central concept of preventive cardiology is that early identification of CHD risk factors and treatment of the associated risk will result in improved survival. Many risk factors begin accumulating at a young age, often while individuals are asymptomatic.2 In fact, pathologic evidence of atherosclerosis can be identified as early as the second and third decades of life,3,4 with even higher prevalence in those with multiple risk factors.5 In some studies, risk factors measured during youth predict atherosclerosis better than those emerging during adulthood.6 For these reasons, the focus of preventive cardiology must be centered on youngsters, adolescents, and young adults because the global epidemic of childhood obesity and diabetes mellitus threatens to reverse the gains that have occurred over the past several decades.
The core science of preventive cardiology is clinical epidemiology. Observational data from prospective cohort studies and interventional data from randomized controlled trials (RCTs) underlie modern risk assessment and preventive care. Beginning in the late 1940s, the Framingham Heart Study advanced the notion of traditional “risk factors” and identified smoking, hypertension, and elevated cholesterol as the principal predictors of CHD.7 Since that time, several landmark pathology-based and population-based studies have elucidated the natural history of atherosclerosis, identified novel risk factors, and expanded our understanding of this condition in populations of varied age and ethnicity (Tables 51–2 and 51–3).
| Study Name | Year Started | Pathology Population Size | Concept |
|---|---|---|---|
| Coronary Disease Among U.S. Soldiers Killed in Action in Korea | 1953 | 300 | Studied coronary arteries during autopsies of young US soldiers killed during the Korean War |
| Coronary Artery Disease in Combat Casualties in Vietnam | 1971 | 105 | Postmortem coronary angiography and dissection of hearts from young US soldiers killed in the Vietnam War |
| Bogalusa Heart Study | 1978 | 204 (93) | Conducted autopsies on young patients age 2-38 y dying of trauma in Bogalusa, Louisiana |
| Pathological Determinants of Atherosclerosis in Youth study (PDAY) | 1987 | 2876 | Collected arteries, blood, tissue, and clinical data from persons ages 15-34 y who died of accidents, homicides, and suicides within 72 h after injury; performed autopsies within 48 h after death |
| Study Name | Year Started | Population Size | Number of Recruitment Centers | Concept |
|---|---|---|---|---|
| Framingham Heart Study | 1948 | 5209 | 1 | Enrolled asymptomatic patients with no CHD history, ages 30-62 y, living in Framingham, Massachusetts |
| Framingham Offspring Study | 1971 | 5124 | 1 | Enrolled offspring of original Framingham cohort to study determinants of CHD in young adults |
| The National Health and Nutrition Examination Survey (NHANES I-IV) | 1971 | ∼5000 per year | 15 counties per year | Enrolled representative survey cohort to model the entire US population (all ages) |
| Coronary Artery Risk Development in Young Adults (CARDIA) | 1986 | 5115 | 4 | Enrolled young adults (ages 18-30 y) to determine early determinants of CHD |
| Atherosclerosis Risk in Communities (ARIC) | 1987 | 15,792 | 4 | Investigated the causes and natural history of atherosclerosis, CHD risk factors, and medical care for new CHD by race, gender, location, and date |
| The Strong Heart Study | 1988 | 4500 | 3 | Because little known about CHD in American Indians, enrolled this population to describe unique determinants of disease |
| Cardiovascular Health Study (CHS) | 1989 | 5888 | 4 | Enrolled older adults (age > 65 y) to describe determinants of CHD in elderly individuals |
| Jackson Heart Study | 1999 | 5302 | 1 | Because there is a greater prevalence of CHD among African Americans, enrolled this population to study reasons for disparity and approaches to reduce it |
| The Multi-Ethnic Study of Atherosclerosis (MESA) | 2000 | 6814 | 6 | Enrolled a multi-ethnic population to describe the pathophysiology of subclinical CHD development and progression and role in clinical CHD |
| The Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) | 2000 | 1214 | 1 | Because the genetically isolated Alaskan Eskimos are rapidly changing from a traditional diet and lifestyle, enrolled this population and genotype their typically large families to describe lifestyle and genetic determinants of CHD |
| The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) | 2008 | 16,000 (target) | 4 | Enrolled Hispanic or Latino populations to determine the role of acculturation and risk factors in the prevalence and development of CHD |
Most of the important modifiable risk factors for CHD have now been identified. Results from the global INTERHEART study suggest that nine risk factors—dyslipidemia, smoking, diabetes mellitus, hypertension, abdominal obesity, psychosocial stress, poor diet, physical inactivity, and reduced alcohol consumption—account for more than 90% of the risk for a first MI (Table 51–4).8 Importantly, the impact of these risk factors appears to be remarkably stable across gender, race, and geographic location. Based on these data, the World Health Organization estimates that 80% of premature CHD can be prevented with comprehensive assessment and early management of these risk factors.9
| INTERHEART: A Global Case-Control Study of Risk Factors for Acute Myocardial Infarction | ||
|---|---|---|
| Risk Factor | Odds Ratio (99% CI) Multivariable Adjusted | Population Attributable Risk Multivariable Adjusted (%) |
| Apo B/Apo A-I | 3.25 (2.82-3.76) | 49 |
| Current smoking | 2.87 (2.58-3.19) | 36 |
| Diabetes | 2.37 (2.07-2.71) | 9.9 |
| Hypertension | 1.91 (1.74-2.10) | 18 |
| Abdominal obesity | 1.62 (1.45-1.80) | 20 |
| Psychosocial stress and depression | 2.67 (2.21-3.22) | 33 |
| Daily fruit and vegetable intake | 0.70 (0.62-0.79) | 14 |
| Exercise | 0.86 (0.76-0.97) | 12 |
| Alcohol intake | 0.91 (0.82-1.02) | |
| Combined | 129 | 90 |
The Relative Benefit of Prevention Compared with Coronary Intervention
Over the past 2 decades, there has been a drastic change in our understanding of chronic CHD. For many years, the pathophysiology was thought to be related to gradual, irreversible plaque growth, leading to progressive obstruction of the coronary artery lumen, and eventual impairment of blood flow. Based on this model, CHD events were thought to be directly related to the degree of luminal obstruction noted on coronary angiography.10
It is now known that luminal obstruction is a relatively late phenomenon in atherosclerosis. Early in the disease process, plaque expansion is accompanied by “positive remodeling,” during which outgrowth of the vessel wall attempts to help preserve luminal diameter (Glagov phenomenon11). When coupled with arterial inflammation and endothelial dysfunction, these remodeled arteries are particularly susceptible to plaque instability.12
An acute coronary syndrome (ACS) results when the fibrous cap of an atherosclerotic plaque fissures, erodes, or ruptures, exposing the lipid core and other prothrombotic contents to the bloodstream. This is followed by variable degrees of coronary thrombosis and reduced coronary perfusion. Although this process was previously thought to occur more commonly at sites of greater stenosis, it is now known that only ˜15% of culprit lesions in MIs have a luminal stenosis greater than 70% before plaque rupture.13,14
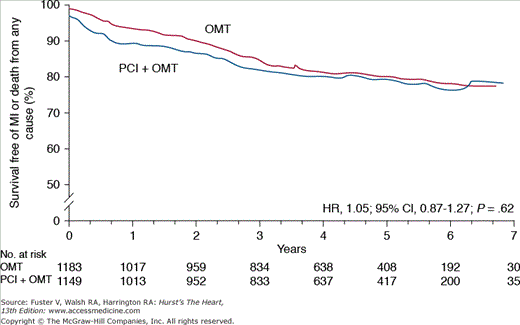
Numerous studies over the past 2 decades have confirmed that routine revascularization of severe stenoses provides no mortality benefit in patients with chronic CHD.15 This paradigm received renewed attention after publication of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial,16,17 which randomized 2287 patients with significant coronary stenosis and stable angina to aggressive medical and lifestyle therapy with or without percutaneous coronary intervention (PCI). After a follow-up of 5 years, there was no difference in anginal symptoms, ACS, or overall mortality with aggressive medical and lifestyle treatment alone, supporting the notion that elective PCI offers little incremental benefit in plaque stabilization (Fig. 51–1).
Figure 51–1.
Primary results of the COURAGE trial—Optimal Medical Therapy (OMT) and percutaneous coronary intervention (PCI) are associated with similar survival over 5-year mean follow-up. CI, confidence interval; HR, hazard ratio; MI, myocardial infarction. Adapted from Boden et al.16
Several important studies have attempted to quantify the relative contribution of risk factor reduction versus revascularization in reducing CHD mortality in the United States. In a recent study, it was estimated that nearly half of the decline in CHD deaths from 1980 to 2000 resulted from population-wide risk factor reduction (44%) and half from evidence-based medical therapy directed at patients with known or suspected vascular disease (47%).18 Importantly, just 10% of the overall reduction was accounted for by interventional therapy in ACS and 5% by revascularization in chronic stable angina.
Similar results have been noted in countries outside of the United States, including an impressive 76% reduction in CHD mortality caused by population-wide risk factor reduction in Finland (Fig. 51–2). Collectively, these studies suggest that most mortality reduction in CHD is caused more by prevention rather than by advanced coronary revascularization procedures.
Figure 51–2.
Estimated decrease in coronary heart disease deaths from population-based risk factor reduction versus individual-based risk factor reduction and acute revascularization therapy. From Ford et al.18
Prevention of CHD occurs at three levels—primordial prevention, primary prevention, and secondary prevention. Each of these differs in the target population, the setting in which care is provided, and the mechanisms of care delivery (Table 51–5).
| Primordial | Primary | Secondary | |
|---|---|---|---|
| Target Patients | All patients, including pediatric | Patients at increased CVD risk | Patients with known CVD |
| Setting | Community, societal | Outpatient | Inpatient → outpatient |
| Delivery of care | Policy decisions Dietary patterns Education campaigns Environment | Education campaigns Behavioral Intervention Medications | Behavioral intervention Medications Rehabilitation |
| Advantages | Intervention before risk factors develop Sustainable Does not require screening | Directed at higher risk individuals Tailored therapy Patients more motivated for change | Directed at highest risk individuals Tailored therapy Patients most motivated for change |
| Disadvantages | Difficult to implement Hard to quantify impact Up-front costs Small individual risk reduction | Requires screening of population May delay, not prevent, disease “Medicalization” of asymptomatic individuals | Small segment of population eligible Attempts to attenuate loss of quality of life Not sustainable |
The term primordial prevention was first coined by Strasser in 1978 and describes efforts to prevent the development of CHD risk factors in a population.19 Primordial prevention occurs predominantly at the societal and community levels and includes policy decisions that influence dietary patterns, educational objectives, and the environment. The principal advantage of primordial prevention is the ability to intervene before the onset of a given risk factor and its associated adverse consequences.
Primordial prevention offers the possibility of sustainable gains in cost-effective care and quality of life without the need for screening and thus is available to all individuals. The principal disadvantage of primordial prevention is that it is difficult to implement effectively on a population-wide basis.
Primary prevention describes efforts to prevent adverse events, such as MI and stroke, in individuals with known risk factors for CHD. This commonly takes the form of individualized lifestyle interventions, including diet and exercise, as well as pharmacotherapy aimed at risk factor reduction. The principal advantage of primary prevention is the ability to tailor therapy to higher risk individuals before the development of clinically significant atherosclerotic disease. The main disadvantage is the requirement for screening of a large segment of the population to identify those at increased risk, which may be both expensive and inexact.
Secondary prevention describes efforts to prevent additional CHD events and CV mortality among patients with known CHD. Most commonly, this involves individualized lifestyle interventions, medications, and cardiac rehabilitation. The principal advantage of secondary prevention is the large relative risk reduction (RRR) that can be achieved within a short period of time given the higher baseline risk of the treated population. The principal disadvantage of secondary prevention is that it is not sustainable as a sole management strategy. Without primordial and primary prevention to reduce the risk factor burden, the cost of secondary prevention in an increasingly obese, aging population is likely prohibitive.
Although the three levels of prevention are generally regarded as distinct, there may be variable degrees of overlap. For example, advanced subclinical atherosclerosis identified with imaging techniques occupies an uncertain middle ground between primary and secondary prevention. This has prompted some to coin the term primary-and-a-half prevention to address this kind of prevention.20
Who should be targeted for preventive therapy? Traditionally, prevention strategies have been divided into those that are population based and those that are individual based (Fig. 51–3).
Population-based prevention seeks to make small changes in risk factors across an entire population with the belief that this will result in large downstream reductions in overall CHD mortality. Such a strategy attempts to produce a “leftward shift” in the risk factor curve for the entire population. Some examples include limiting public smoking, trans fat bans, and educational campaigns aimed at reducing saturated fat and salt intake (Table 51–6).
| Modified 2006 AHA Population-Based Recommendations for CHD Reduction |
|---|
| • Balance calorie intake and physical activity to achieve a healthy body weight. |
| • Consume a diet rich in vegetables and fruits. |
| • Choose whole-grain, high-fiber foods. |
| • Consume fish, especially oily fish, at least twice a week. |
| • Limit saturated fat to <7% of energy and cholesterol <300 mg. |
| • Minimize trans fat to <1% of energy or eliminate. |
| • Limit sugar intake to <100 kcal/d for women and <150 kcal/d for men. |
| • Choose and prepare foods with little or no salt. |
| • Follow AHA recommendations when eating outside of the home. |
| • If alcohol is consumed, do so in moderation. |
| • Stop smoking and ensure a smoke-free environment. |
| • Expedited adoption of National Ambient Air Quality Standards. |
Individual-based prevention attempts to target high-risk patients for aggressive, individualized therapy. Such a strategy relies on risk assessment tools to accurately identify those at highest risk because these patients are most likely to benefit from individualized therapy. One example is the use of cholesterol lowering therapy based on guidelines from the National Cholesterol Education Program Adult Treatment Panel (NCEP ATP).
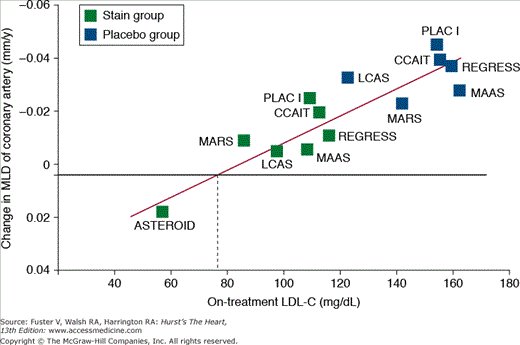
Historically, the goal of clinical preventive therapy has been to normalize abnormal risk factors. Data from several recent studies, however, have challenged this notion, suggesting an even greater benefit by modifying risk factors to levels below what is considered “normal.” In the case of cholesterol management, administration of aggressive lipid-lowering therapy to higher risk individuals with “normal” cholesterol levels has been associated with reduced CV risk and may contribute to slowing of atherosclerotic progression, plaque stabilization, and even regression of atherosclerosis26,27 (Fig. 51–4). This has resulted in a paradigm shift in which aggressive risk-reducing therapy is administered to all high-risk individuals rather than solely focusing on correction of a specific abnormal risk factor in a given individual.
Figure 51–4.
Association between low-density lipoprotein cholesterol (LDL-C) and progression and regression of coronary atherosclerosis. MLD, minimum lumen diameter. From Ballantyne et al.27
This was well illustrated in the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) study,28 which randomized patients with angiographically established coronary artery disease to high-dose atorvastatin or moderate dose pravastatin. In the atorvastatin arm, low-density lipoprotein cholesterol (LDL-C) lowering to levels well below what was considered “normal” led to a halt of atherosclerotic progression. Similar findings were noted in the Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID), in which treatment with rosuvastatin was associated with significant LDL-C reduction and modest mean regression of total plaque volume as measured by intravascular ultrasound (IVUS).29
The clinical benefit of such approaches was validated by the Myocardial Ischemia Reduction with Acute Cholesterol Lowering (MIRACL) trial, which demonstrated the ability of high-dose atorvastatin initiated early after an MI to reduce near-term adverse events.30 Based on these data, many have called into question whether some “normal” cutpoints for specific risk factors are in fact normal.
Perhaps nowhere else in medicine is there more robust evidence than that which causally links CV risk factors to CHD. Despite this, numerous gaps exist today in our ability to appropriately identify at-risk individuals and to initiate risk-reducing therapies, even among those deemed to be at highest risk.
In a previous survey of 48,586 outpatients with CHD from 140 medical practices (80% cardiology), only 39% were treated with lipid-lowering medication, and 11% were documented to have LDL-C levels below 100 mg/dL.31 In a subsequent national survey of 4885 outpatients, only 67% achieved their LDL-C treatment goal overall, with control rates of 89%, 76%, and 57% among those with no or one CHD risk factor, more than two CHD risk factors or established CHD, and a CHD risk equivalent, respectively.32
Control rates were even lower among those with diabetes mellitus (55%) and those at very high risk, with a LDL-C goal below 70 mg/dL (18%). Thus, at a time when there is greater availability of risk-reducing therapies than at any time before, it is difficult to accept that recent data from the National Health and Nutrition Examination Survey (NHANES) indicates that just one in 12 adults are at low CHD risk.33
Numerous reasons exist for the treatment gap in CV prevention. One important component is variability in patient adherence. Data from the Duke Databank for Cardiovascular Disease indicate that although use of evidence-based therapies among CHD patients is increasing, adherence to these medications even among a select population with angiographically proven coronary artery disease is largely poor (71% for aspirin, 20% for angiotensin-converting enzyme inhibitors [ACEIs], 46% for β-blockers, and 44% for lipid-lowering therapy). Cultural, socioeconomic, and geographic differences among patient populations likely contribute to this, both by affecting access to healthy lifestyles as well as access to quality health care.34
From a provider standpoint, lower utilization of risk-reducing therapies may be caused by an unfounded belief by some that patients enrolled in clinical trials are dissimilar from those encountered in typical clinical practice. For others, there is thought to be insufficient time to address preventive practices during a busy work day despite their acknowledged importance. Support for this comes from previous estimates that complete delivery of U.S Preventive Services Task Force (USPSTF) CHD guidelines would add at least 1.5 hours to a clinical workday.35 Still, for others, there is less implementation of certain therapies because of a belief that the guidelines are too complex and cumbersome.
Finally, at the societal and organizational level, limited reimbursement for prevention remains a major barrier to instituting preventive care. Although clinical studies confirm that preventive care increases quality of life and overall survival and may be cost saving, reimbursement remains substantially higher for imaging studies and invasive procedures, which provide less incremental benefit and in some cases may be harmful.36Accordingly, from a public health perspective, it is important that economic incentives be aligned with evidence-based guidelines and evidence-based guidelines be simplified for the most effective implementation.
Many textbooks, scientific reviews, and lay press publications have attempted to summarize all that is included in CHD prevention within a single document. These papers are often difficult to read, difficult to implement, and thus of limited use in the clinical setting.
Over the past 10 years, the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease has pioneered implementation of the “ABCDE” approach to preventive cardiology.37 Recognizing simplicity as a central key to guideline adherence, this approach organizes guideline recommendations into an easy-to-remember memory tool that can be used by providers and patients alike. Although for any given patient, only select components of the approach may be applicable, the ABCDE approach ensures that no aspects of comprehensive preventive care are missed. We have chosen to organize the remainder of the chapter using this format (Table 51–7).
| A | Assessment of riska |
| Antiplatelet therapy | |
| Anticoagulation therapy | |
| B | Blood pressure |
| C | Cholesterol |
| Cigarette smoking cessation | |
| D | Diet and weight management |
| Diabetes prevention and treatment | |
| E | Exercise |
Assessment
The development of CHD is a complex, multifactorial process in which genetics, gender, risk factors, and aging play varying roles in predisposing susceptible patients to the development of atherosclerosis38 (Fig. 51–5). Over the past 20 years, there has been increased recognition that multiple CHD risk factors produce a synergistic effect that is associated with increased CHD risk. This has shifted focus away from individual risk factors as the principal determinant of treatment, with greater attention toward global assessment of CHD risk.39 This concept is particularly important because patients may warrant aggressive preventive therapy even when individual risk factors are not particularly abnormal.
Accurate risk assessment is the cornerstone of cost-effective individual-based preventive care.40 At the clinical level, the intensity of treatment should correspond to one’s individual risk. This approach is not only logical but would be expected to produce the largest RRR, the smallest number needed to treat, (NNT) and therefore the lowest cost per quality adjusted life year (QALY) saved.
Global risk assessment tools that use risk-scoring algorithms are most useful for assigning a low-, intermediate-, or high-risk status to patients who are asymptomatic, nondiabetic, and free of known CHD—the primary prevention population. In contrast, individuals with known CHD, other forms of vascular disease, and diabetes mellitus are at high risk, with little to be gained by these risk assessment tools.
The Framingham Risk Score (FRS) represents the most widely used CHD risk assessment tool.41 Originally derived from the Framingham Heart Study, this risk score is designed to calculate the 10-year risk of an initial MI or CHD-related death by using age, the total cholesterol level, the high-density lipoprotein cholesterol (HDL-C) level, smoking status, and systolic blood pressure to estimate risk in asymptomatic men and women free of known CHD. Patients are then stratified into low- (<10% 10-year risk), intermediate- (10%-20% 10-year risk), or high-risk (>20 % 10-year risk) groups. Although the FRS has been validated in several external populations,42,43 it does not include some other important risk factors that have been shown to predict CHD events in several large studies.44
The FRS currently forms the backbone of the NCEP ATP guidelines for the clinical management of patients with cholesterol.45 It also serves as the reference against which other prospective risk factors are tested. In fact, new risk factors are generally considered to have limited clinical utility if they do not enhance risk discrimination or change risk classification compared to the FRS.46
When it has been applied to different populations, some have proposed that the FRS be multiplied by “prevalence correction factors.”42,47 Others have proposed the creation of new risk scores altogether, such as the Systematic Coronary Risk Evaluation (SCORE) project in Europe48 and the QRESEARCH cardiovascular RISK (QRISK) algorithm in the United Kingdom.49 In contrast to the FRS, which was derived from a sample of &tild;5,000 patients, the QRISK score came from a primary care database of more than 10 million patients followed over a period of 17 years.
Despite its tremendous value in predicting CHD events and mortality, the traditional FRS does not assess the risk of other clinically important CV events such as stroke, peripheral arterial disease (PAD), and heart failure. It has been proposed that this may be one reason why the FRS underestimates overall CV risk in several important populations.50
In response to these limitations, a revised and more comprehensive FRS for the prediction of adverse CV outcomes was introduced in 2008.51 Using two separate risk-scoring methods based on whether data is collected during a general office visit (incorporating age, body mass index [BMI], blood pressure, treatment of hypertension, smoking, and diabetes) or from laboratory studies (including HDL-C and total cholesterol levels), this simplified algorithm is easily calculated in the office setting50 (Tables 51–8 and 51–9).
| Points | Age (y) | BMI (kg/m2) | SBP Untreated (mm Hg) | SBP Treated (mm Hg) | Smoker? | Diabetic? | CVD Risk (%) | Heart Age (y) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| –3 | <120 | 1 | <1 | <30 | <30 | |||||||||||
| –2 | <120 | 1 | <1 | <30 | <30 | |||||||||||
| –1 | <120 | No | No | No | No | 2 | 1 | 31 | <30 | |||||||
| 0 | 30-34 | 30-34 | <25 | <25 | 120-129 | 120-129 | <120 | 2 | 1 | 33 | 32 | |||||
| 1 | 25-30 | 25-30 | 130-139 | 130-139 | 3 | 2 | 35 | 34 | ||||||||
| 2 | 35-39 | 35-39 | >30 | >30 | 140-159 | 120-129 | 120-129 | 3 | 2 | 37 | 36 | |||||
| 3 | >160 | 140-149 | 130-139 | 130-139 | Yes | 4 | 2 | 39 | 38 | |||||||
| 4 | 150-159 | 140-150 | 140-149 | Yes | Yes | 5 | 3 | 41 | 41 | |||||||
| 5 | 40-44 | 40-44 | >160 | >160 | 150-159 | Yes | 6 | 3 | 44 | 43 | ||||||
| 6 | 45-49 | 7 | 4 | 46 | 46 | |||||||||||
| 7 | 45-49 | >160 | 8 | 4 | 49 | 48 | ||||||||||
| 8 | 50-54 | 50-54 | 10 | 5 | 52 | 51 | ||||||||||
| 9 | 11 | 5 | 55 | 54 | ||||||||||||
| 10 | 55-59 | 55-59 | 13 | 6 | 58 | 58 | ||||||||||
| 11 | 60-64 | 60-64 | 15 | 7 | 62 | 61 | ||||||||||
| 12 | 65-69 | 18 | 8 | 65 | 65 | |||||||||||
| 13 | 65-69 | 22 | 10 | 69 | 69 | |||||||||||
| 14 | 70-74 | 70-74 | 25 | 11 | 73 | 73 | ||||||||||
| 15 | >75 | >75 | 29 | 13 | 78 | 77 | ||||||||||
| 16 | >30 | 15 | 80 | 80 | ||||||||||||
| 17 | >30 | 18 | >80 | >80 | ||||||||||||
| 18 | >30 | >20 | >80 | >80 | ||||||||||||
| 19 | >30 | >24 | >80 | >80 | ||||||||||||
| 20 | >30 | 27 | >80 | >80 | ||||||||||||
| >20 | >30 | >30 | >80 | >80 | ||||||||||||
| Points | Age (y) | HDL-C (mg/dL) | Total C (mg/dL) | SBP Untreated (mm Hg) | SBP Treated (mm Hg) | Smoker? | Diabetic? | CVD Risk (%) | Heart Age (y) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| –3 | <120 | <1 | <1 | <30 | <30 | |||||||||||||
| –2 | >60 | >60 | <120 | 1 | <1 | <30 | <30 | |||||||||||
| –1 | 50-59 | 50-59 | <120 | 1 | 1 | <30 | <30 | |||||||||||
| 0 | 30-34 | 30-34 | 45-49 | 45-49 | <160 | <160 | 120-129 | 120-129 | <120 | No | No | No | No | 1 | 1 | 30 | 30 | |
| 1 | 35-44 | 35-44 | 160-199 | 160-199 | 130-139 | 130-139 | 2 | 1 | 32 | 31 | ||||||||
| 2 | 35-39 | 35-39 | <35 | <35 | 200-239 | 140-159 | 140-149 | 120-129 | 120-129 | 2 | 2 | 34 | 34 | |||||
| 3 | 240-279 | 200-239 | >160 | 130-139 | 130-139 | Yes | 3 | 2 | 36 | 36 | ||||||||
| 4 | 40-44 | >280 | 240-279 | 150-159 | 140-159 | Yes | Yes | Yes | 3 | 2 | 38 | 39 | ||||||
| 5 | 40-44 | 45-49 | >280 | >160 | >160 | 140-149 | 4 | 3 | 40 | 42 | ||||||||
| 6 | 45-49 | 150-159 | 5 | 3 | 42 | 45 | ||||||||||||
| 7 | 50-54 | >160 | 6 | 4 | 45 | 48 | ||||||||||||
| 8 | 50-54 | 55-59 | 7 | 4 | 48 | 51 | ||||||||||||
| 9 | 60-64 | 8 | 5 | 51 | 55 | |||||||||||||
| 10 | 55-59 | 65-69 | 9 | 6 | 54 | 59 | ||||||||||||
| 11 | 60-64 | 70-74 | 11 | 7 | 57 | 64 | ||||||||||||
| 12 | 65-69 | >75 | 13 | 9 | 60 | 68 | ||||||||||||
| 13 | 15 | 10 | 64 | 73 | ||||||||||||||
| 14 | 70-74 | 18 | 12 | 68 | 79 | |||||||||||||
| 15 | >75 | 21 | 14 | 72 | 80 | |||||||||||||
| 16 | 25 | 16 | 76 | >80 | ||||||||||||||
| 17 | 29 | 18 | >80 | >80 | ||||||||||||||
| 18 | >30 | 22 | >80 | >80 | ||||||||||||||
| 19 | >30 | 25 | >80 | >80 | ||||||||||||||
| 20 | >30 | 29 | >80 | >80 | ||||||||||||||
| >20 | >30 | >30 | >80 | >80 | ||||||||||||||
By incorporating the concept of “vascular” or “biologic” age, the revised FRS is also more readily understood by patients.52,53 This is achieved by attempting to equate the age of an at-risk individual to another individual without any risk factors who has the same 10-year risk of adverse CV events. For example, it may be easier for a 50-year-old patient to understand that he has the CV system of a healthy 70-year-old as opposed to a 20% statistical probability of a CV event over the next 10 years.
Although the benefits of the revised FRS seem obvious, it has not yet been incorporated into current guidelines for the treatment of cholesterol or other risk factors. In addition, it remains to be determined how treatment of an individual with a 10% 10-year CHD risk with the traditional FRS should compare with treatment of a similar individual with a 15% to 30% 10-year CV risk using the revised FRS. Nonetheless, even if the revised FRS falls short as a superior risk stratification tool, it may provide incremental gains if it helps improve compliance with risk-reducing therapy.
Another limitation of the traditional FRS is its inability to estimate risk beyond 10 years. Because age has a disproportionate effect on the calculated FRS, a younger patient with several risk factors is much less likely to qualify for aggressive risk-reducing therapy.
This is well illustrated by a 50-year-old asymptomatic man who does not smoke and has a total cholesterol level of 220 mg/dL, an HDL-C level of 39 mg/dL, and a systolic blood pressure of 132 mm Hg on blood pressure-lowering therapy. Using the FRS, 8% or eight of 100 individuals similar to this patient would be expected to experience a CHD event over the next 10 years, placing this individual in the low-risk category without a recommendation for lipid-lowering therapy. Two years later, without any change in the risk factor burden, the patient is now in the intermediate-risk category with a 10-year risk of 10%, which qualifies the patient for aspirin and lipid-lowering therapy. By age 65 years, with no change in the risk factor burden, the FRS is now 20%, placing the patient at high risk.
Based on just such an example, it has been argued by many that certain risk-reducing therapies (eg, aspirin and lipid-modifying therapy) should be instituted earlier without waiting for the calculated risk to match that which is believed to be clinically prudent. Because atherosclerosis is a lifelong disease, it may be reasonable to estimate the CV risk for a group of patients over their remaining lifetimes and factor this into treatment decisions.54 For example, although the 10-year risk of CHD is generally low for men and women at age 50 years, nearly one in two men and one in three women in this age group will develop CHD during their lifetime.
The traditional FRS does not reliably identify individuals with low short-term but high lifetime risk for CHD.55 Recent studies have demonstrated, however, that these patients have a substantial burden of subclinical atherosclerosis.56 This is not surprising to many because a suboptimally treated risk factor portends a higher likelihood of developing symptomatic CHD even if it does not occur during the next 10 years.
To address these limitations, the Framingham Heart Study investigators recently published a risk score for predicting CV risk over 30 years.57 Traditional risk factors were identified as continued strong predictors of CV disease in this model. Obesity was identified as a stronger predictor of events in the long term compared with the short term and is likely related to an association of obesity and a worsening risk factor profile as one ages.
According to NHANES III, fewer than 1% of asymptomatic women are classified as high risk using the traditional FRS and only &tild;4% of women without diabetes mellitus have a 10-year risk of 10% to 20%.58 Accordingly, only a small fraction of women are eligible for aspirin and lipid-modifying therapy.59 At odds with this, however, is the fact that after reaching age 50 years, about 40% of women will have a CV event during their lifetime.60
Women typically have higher levels of C-reactive protein (CRP) than men,61 suggesting that measurement of this acute phase reactant may be of benefit. The high-sensitivity assay for CRP (hsCRP) is an important measure of subclinical inflammation that is associated with increased CV risk and has been shown to provide prognostic value independent of traditional risk factors and improve the risk stratification of individuals compared with the FRS.62
The greatest value of hsCRP to date was demonstrated in the Justification for the Use of statins in Primary prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial.63 This study randomized nearly 18,000 men older than 55 years and women older than 65 years with a LDL-C below 130 mg/dL and a hsCRP of 2 mg/L or above to rosuvastatin (20 mg/d) or placebo in an attempt to evaluate whether elevated levels of hsCRP could identify a group of individuals (mean LDL-C, 104 mg/dL) who would benefit from lipid-modifying therapy despite having LDL-C levels at the desired goal. This trial was stopped early after a mean follow-up of just 1.9 years because of a 47% reduction in major CHD events and a 20% reduction in all-cause mortality among those treated with rosuvastatin.
Historically, family history of premature CHD has been omitted from traditional risk scoring even though many risk factors are partly heritable.64-66 Recent studies have confirmed that offspring of parents with CHD are at increased risk,67 and this risk may be even higher if there are affected siblings.68 Within the Framingham Offspring cohort, there was a two-fold increase in CHD risk among individuals with a family history of premature CHD.69
Investigators from the Multi-Ethnic Study of Atherosclerosis (MESA) study have also shown a substantial increased risk of subclinical atherosclerosis in patients of varying ethnicities with a family history of premature CHD.70 Not surprisingly, almost 75% of patients with premature CHD have a family history of premature CHD.71,72
Given the limitations above, the Reynolds Risk Score (RRS) was developed as an alternate means to assess global CV risk in healthy women. In addition to traditional risk factors, this risk score assesses both levels of hsCRP and the presence of a family history of premature CHD.73 When it was applied to nearly 25,000 healthy women from the Women’s Health Study, approximately 40% of women previously estimated to be at low or moderate risk using traditional risk estimation would be reclassified to higher or lower risk categories. Similar findings have been noted in men, with the RRS reclassifying &tild;20% of men into higher or lower risk categories among a cohort of 10,724 healthy men.74
Increased caloric intake, greater consumption of refined carbohydrates, and physical inactivity have contributed to dramatic increases in the incidence of abdominal obesity and the emerging epidemic of insulin resistance. The term metabolic syndrome has been used to describe the clustering of individual risk factors—including atherogenic dyslipidemia, glucose intolerance, elevated blood pressure, a proinflammatory state, and a prothrombotic state—that result from abdominal obesity and insulin resistance.
Although historically there has been a great deal of controversy regarding whether the term metabolic syndrome is in fact a true clinical syndrome, it does effectively alert physicians to an important patient phenotype.75 Specifically, metabolic syndrome is associated with a greater than would be expected risk for diabetes mellitus, subclinical atherosclerosis, and subsequent CV events, especially among individuals classified as low risk by the FRS.
Although metabolic syndrome is not a risk-scoring instrument, its diagnosis can be used to upwardly modulate the risk predicted by traditional models (Table 51–10). This has prompted some to consider extending the intermediate-risk group to include those with a 6% to 20% 10-year CHD risk.76 Physicians could then use this adjusted risk categorization to guide more aggressive lifestyle changes; lower blood pressure goals; and initiate therapy with aspirin, LDL-C modifying medications, and renin–aldosterone–angiotensin system (RAAS) blockers at an earlier time point than would conventionally be recommended.
| WHO, 1999 | NCEP ATPIII, 2001 | IDF, 2005 |
|---|---|---|
Insulin resistance
| At least three of the following five criteria:
Triglycerides ≥150 mg/dL
Hypertension ≥130/85 mm Hg Fasting glucose ≥100 mg/dL | Abdominal obesity Identified as:
|
|
|
Although traditional risk factors are helpful in identifying patients who might be at risk for CHD, measures of subclinical atherosclerosis provide a more direct measure of individualized CHD burden and thus integrate the effect of lifetime exposure to measured and unmeasured risk factors. Detection of subclinical atherosclerosis can also be used to enhance patient motivation and guide the intensity of therapy based on individualized disease burden.
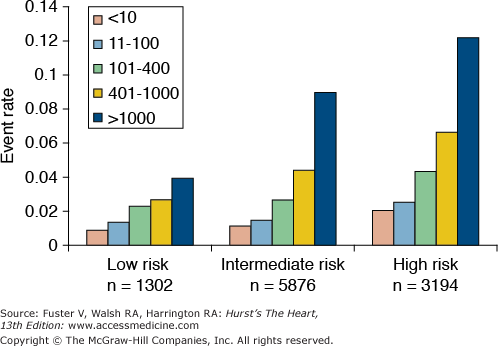
The two most established clinical measures of subclinical atherosclerosis are carotid intima–media thickness (cIMT) measured by B-mode ultrasonography, and coronary artery calcium (CAC) measured by computed tomography (CT). Although both measures have been shown to predict CHD events after adjustment for traditional risk factors,77,78 CAC outperforms cIMT in its ability to predict CHD in middle-aged and older adults.79 High levels of CAC can be helpful in reclassifying lower risk patients,80,81 and the absence of CAC is associated with excellent survival and may be used to select patients in whom certain forms of pharmacotherapy are not needed82 (Fig. 51–6).
Figure 51–6.
The coronary artery calcium score predicts coronary heart disease beyond the Framingham Risk Score and helps reclassify patients into different risk categories. From Shaw et al.80
The ankle-brachial index (ABI), which traditionally has been used to evaluate the severity of PAD, is another marker of subclinical vascular disease that rarely is abnormal in persons younger than 60 years of age who do not have a history of smoking or diabetes.83 Exercise stress testing has also been used for risk stratification and may be of greatest value in persons with multiple cardiac risk factors who want to start a vigorous exercise program.84
Assessment of subclinical atherosclerosis is most appropriate in patients at intermediate risk, in whom treatment decisions are most uncertain.85 This initially led the American College of Cardiology (ACC) Bethesda conference on atherosclerosis imaging to endorse coronary calcium scoring in an expanded intermediate-risk category (6%-20% 10-year risk of a hard CHD event) to help in predicting risk not fully captured by the FRS.86 In 2006 and 2007, the American Heart Association (AHA) followed by the ACC/AHA joint guidelines considered these tests “reasonable” options for risk stratification in intermediate-risk patients (class II recommendation, Level of Evidence: B).87 Most recently, the Society for Heart Attack Prevention and Eradication (SHAPE) has taken this one step further, with consideration of testing for subclinical atherosclerosis in all men older than age 45 years and women older than age 55 years who do not already qualify for aggressive risk-reducing therapy.38
Despite numerous improvements in risk scoring, there still remain patients identified as being low risk who experience CHD events, as well as patients deemed high risk who remain free of CHD events. This has led to a search for additional risk variables that may aid in further risk discrimination.
Table 51–11 summarizes the use of many of these emerging risk variables that may be measured in asymptomatic, primary prevention patients.88 Various barriers exist, however, to the use of these emerging variables, limiting their more widespread use. In some cases, the tests are not universally available (apolipoprotein B) or are relatively expensive (lipoprotein-associated phospholipase A2 [Lp-PLA2]). In other cases, the tests harbor potential risk (radiation with myocardial perfusion imaging or cardiac CT) or require specialized laboratories for testing (brachial flow-mediated dilation and genetic testing).
| Test | Rationale | Recommendation Class and LOE | Limitation |
|---|---|---|---|
| Genetic testing (eg, 9p21.3 SNP) | Genetic variants have been associated with increased risk | III, B | Testing not widely available; expensive |
| Advanced lipoprotein testing (eg, apo B) | Apo B, LDL particle number, and particle size may outperform traditional lipid parameters | III, C | Several different testing procedures; not standardized |
| Natriuretic peptides (eg, pro-BNP) | Levels are increased with myocardial wall stress | III, B | More helpful for heart failure than CHD |
| Hemoglobin A1c | Integrates glucose control over preceding 3 mo | IIb, B | Not yet part of metabolic syndrome or diabetes definition |
| Microalbuminuria | Correlates with early endothelial dysfunction | IIa or IIb, B | None |
| Lp-PLA2 | Associated with plaque inflammation and plaque rupture | IIb, B | Testing not widely available, expensive |
| Brachial flow-mediated vasodilation (FMD) | Noninvasive measure of endothelial dysfunction | III, B | Operator variability; not widely available |
| Arterial stiffness (eg, pulse wave velocity) | Correlated with aging, hypertension, pulse pressure | III, C | Protocols not well standardized; not widely available |
| Myocardial perfusion imaging | Assess for asymptomatic myocardial ischemia | III, C | High-radiation dose; expensive |
| CT angiography | Noninvasive coronary angiogram and plaque morphology | III, C | Moderately high-radiation dose |
| Noninvasive assessment of plaque morphology and function | III, C | Not widely available; expensive |
Who should be targeted for more aggressive CHD prevention strategies? Should age be a major consideration? Should there be a focus on absolute or relative risk?
Although age is clearly an important predictor of CHD risk, it invites potential ethical dilemmas with regard to screening. Should we predominantly target older individuals because their absolute risk of CHD is much higher? Should we focus our efforts on younger individuals because more QALYs can be gained, more productivity for society can be preserved, and one can intervene earlier in the course of disease?
If absolute risk reduction is of greater importance, are we prepared to focus disproportionate efforts toward men and those with unhealthy behaviors because these populations have higher absolute risk?
Unfortunately, there are no definitive answers to these questions, and clinical judgment on an individual basis remains paramount.89
Antiplatelet Therapy: Aspirin
Initially developed for its antipyretic and antirheumatic properties, aspirin remains the most widely used antithrombotic agent in the world today. Its CV benefit stems from its ability to irreversibly inhibit cyclooxygenase-1 (COX-1), resulting in reduced generation of the potent platelet agonist thromboxane.
A substantial wealth of data supporting the use of aspirin comes from its evaluation in primary prevention. In the largest meta-analysis published to date, the Antithrombotic Trialists’ Collaboration evaluated 95,456 low-risk patients from six clinical trials.90 Individuals were randomized to treatment with placebo or aspirin at varying doses (ranging between 100 mg on alternate days to 500 mg/d) for a duration of 4 to 10 years. Although aspirin therapy was associated with a small but statistically significant reduction in serious vascular events (0.51% vs 0.57% per year; P = .0001), it was also associated with an increase in the rate of major gastrointestinal and extracranial bleeding (0.10% vs 0.07% per year; P <.0001).
More recent clinical trial data, however, have called into question the ischemic benefit of aspirin in certain higher risk primary prevention populations. In the Aspirin for Asymptomatic Atherosclerosis (AAA) trial, 3350 patients ages 50 to 75 years with an ABI of 0.95 or below were randomized to aspirin (100 mg/d) or placebo.91 After a mean follow-up of 8 years, there was no difference in the rate of adverse CV events (hazard ratio [HR], 1.03; 95% confidence interval [CI], 0.84-1.27).
Similar findings were noted in the Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial,92 which randomized 1276 patients with diabetes mellitus and an ABI of 0.99 or below to aspirin (100 mg/d) or placebo. After a median of 7 years, there was no difference in the rate of adverse CV events (18.2% vs 18.3%; 95% CI, 0.76-1.26).
The role of aspirin in patients with diabetes mellitus was also recently evaluated in the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes (JPAD) trial. This study randomized 2539 patients with type 2 diabetes mellitus to low-dose aspirin (81-100 mg/d) or placebo in an open-label study design.93 After a mean of 4.4 years, there was a nonsignificant reduction in the incidence of adverse CV events (HR, 0.80; 95% CI, 0.58-1.10). Among patients 65 years and older, however, there was a significant benefit to treatment with aspirin therapy (HR, 0.68; 95% CI, 0.46-0.99).
The finding that benefit from aspirin was limited to individuals older than 65 years of age was also noted in the Women’s Health Study94 in which 39,876 women ages 45 years and older were randomized to aspirin (100 mg on alternate days) or placebo for a mean of 10 years. Although aspirin was associated with a small, nonsignificant reduction in adverse CV events among all women (HR, 0.91; 95% CI, 0.80-1.03), those ages 65 years and older were noted to have a significant CV benefit (HR, 0.74; 95% CI, 0.59-0.92).
Importantly, there appear to be gender-specific differences in the types of adverse CV events that are prevented with aspirin therapy. This was evaluated recently in a large meta-analysis of 51,342 women and 44,114 men.95 Among women treated with aspirin, there was a 12% reduction in adverse CV events (HR, 0.88; 95% CI, 0.79-0.99), driven predominantly by a reduction in the rate of ischemic stroke (HR, 0.76; 95% CI, 0.63-0.93), with no effect on the rate of MI (HR, 1.01; 95% CI, 0.84-1.21).
In contrast, treatment with aspirin among men resulted in a 14% reduction in adverse CV events (HR, 0.86; 95% CI, 0.78-0.94) driven by a reduction in the rate of MI (HR, 0.68; 95% CI, 0.54-0.86) with no effect on the rate of ischemic stroke (HR, 1.00; 95% CI, 0.72-1.41). Gender-specific differences did not affect the risk of adverse outcomes, as men and women experienced near identical increased rates of major bleeding with aspirin therapy (HR, 1.72; 95% CI, 1.35-2.20 for men and HR, 1.68; 95% CI, 1.13-2.52 for women).
In contrast to the recent discrepant results regarding the role of aspirin therapy in primary prevention, there is a consistent, larger treatment effect favoring aspirin use in secondary prevention. In a large meta-analysis from the Antithrombotic Trialists’ collaboration, &tild;17,000 high-risk patients were randomized to treatment with aspirin or placebo. Aspirin use was associated with a significant reduction in major vascular events (6.7% vs 8.2% per year; P <.0001), stroke (2.1% vs 2.5% per year; P <.002), and coronary events (4.3% vs 5.3% per year; P <.0001), without reduced efficacy at lower doses of aspirin (75-150 mg).96
Traditionally, higher doses of aspirin (162-325 mg/d) have been recommended early after PCI based on the belief that this will attenuate subsequent thrombotic events. The recently presented Clopidogrel optimal loading dose Usage to Reduce Recurrent Events-Organization to Assess Strategies in Ischemic Syndromes (CURRENT OASIS 7) trial, however, calls into question this recommendation.97 This study randomized 25,087 patients with an ACS in a 2 × 2 factorial design to double-dose clopidogrel (600 mg loading dose; 150 mg/d for 7 days; then 75 mg/d thereafter) versus standard-dose clopidogrel (300 mg loading dose; then 75 mg/d thereafter) and high-dose aspirin (300-325 mg) versus low-dose aspirin (75-100 mg) for 30 days, with 70% of patients in this trial undergoing PCI. The primary end point of CV death, MI, or stroke was not different among patients treated in the low- and high-dose aspirin arms (4.4% vs, 4.2%, respectively; 95% CI, 0.85-1.08).
The benefit of aspirin therapy in CV prevention must be weighed against potential bleeding risks.
Primary prevention guidelines:
Aspirin (75-162 mg/d) in those at intermediate risk (10-year risk of CHD ≥10%; ACC/AHA class I, level A)98
Aspirin (81 mg/d or 100 mg every other day) in at-risk women 65 years of age or older (ACC/AHA class IIa, level B), consideration of aspirin in at-risk women younger than 65 years of age for ischemic stroke prevention (ACC/AHA class IIb, level B), and discouraged use of aspirin in optimal-risk women younger than 65 years of age (ACC/AHA class III, level B)99
Aspirin (75-162 mg/d) in those with diabetes mellitus who are older than 40 years of age or who have additional risk factors (family history of premature CV disease, hypertension, smoking, dyslipidemia, or albuminuria) (AHA/ American Diabetes Association [ADA])100
Aspirin (81 mg/d) for men beginning at age 45 years and for women beginning at age 55 years (USPSTF)101
Secondary prevention guidelines:
Aspirin (75-162 mg/d) in those with known CHD or atherosclerotic CV disease (ACC/AHA class I, level A)102
Aspirin (162-325 mg/d) for at least 1 month after bare metal stent implantation, at least 3 months after sirolimus-eluting stent implantation, and at least 6 months after paclitaxel-eluting stent implantation after which aspirin (75-162 mg/d) should be continued indefinitely (ACC/AHA class I, level B)103
Aspirin (75-162 mg/d) as the initial dose after stent implantation in those at higher bleeding risk (ACC/AHA class IIa, level C)103
Aspirin (100-325 mg/d) following coronary artery bypass graft (CABG) surgery (ACC/AHA class I, level B)102
Antiplatelet Therapy: P2y12 Receptor Antagonists
Also known as ADP receptor antagonists or thienopyridines, the P2Y12 receptor antagonists act on platelets to inhibit cAMP-dependent thromboxane generation and activation of the fibrinogen receptor. The downstream effect of these agents is potent inhibition of platelet activation and sustained aggregation.
Most clinical trials evaluating P2Y12 receptor antagonists have attempted to determine the incremental benefit of adding this agent to aspirin (dual antiplatelet therapy) compared with treatment with aspirin alone. Little data exist regarding the use of P2Y12 receptor antagonists alone in primary prevention.
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial randomized a heterogeneous population of 15,603 patients with multiple CV risk factors or known CV disease to aspirin (75-162 mg/d) or aspirin (75-162 mg/d) and clopidogrel (75 mg/d) for a mean of 30 months.104 Although dual antiplatelet therapy was associated with a small trend toward reduced frequency of CV death, MI, or stroke (HR, 0.93; 95% CI, 0.83-1.05) for the entire study population, there was a strong trend toward increased risk of this combined end point among those being treated for primary prevention (HR, 1.2; 95% CI, 0.91-1.59).
One of the first large trials evaluating a P2Y12 receptor antagonist in head-to-head comparison to aspirin was the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.105 This study randomized 19,185 patients with a history of ischemic stroke, MI, or peripheral artery disease to aspirin (325 mg/d) or clopidogrel (75 mg/d) for 2 years. Treatment with clopidogrel was associated with a modest 9% RRR (5% vs 6%; P = .043) in the primary end point of ischemic stroke, MI, or vascular death, with most benefit noted in those individuals with preexisting PAD.
Several large studies subsequent to the CAPRIE trial sought to evaluate the benefit of dual antiplatelet therapy compared with aspirin monotherapy in those with an ACS. The first of these was the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial, which randomized 12,562 patients with a non–ST-segment elevation (NSTE) ACS (NSTE-MI or unstable angina) to aspirin (75-325 mg/d) or aspirin (75-325 mg) plus clopidogrel (300-mg load; 75 mg/d thereafter) for 9 months.106
Dual antiplatelet therapy was associated with a 20% RRR (9.3% vs 11.4%; P <.001) in a composite of death from CV causes, nonfatal MI, or stroke. Dual antiplatelet therapy was also associated with an increased risk of major bleeding (3.7% vs 2.7%; P = .001), particularly when higher dose aspirin was used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree